Alcohols occupy a central position in organic chemistry. They can be prepared from many other kinds of compounds (alkenes, alkyl halides, ketones, esters, and aldehydes, among others), and they can be transformed into an equally wide assortment of compounds (Figure 17.4).
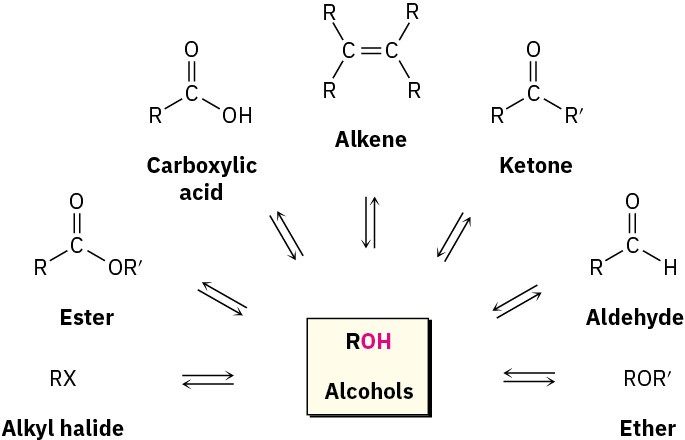
Figure 17.4 The central position of alcohols in organic chemistry. Alcohols can be prepared from, and converted into, many other kinds of compounds.
We’ve already seen several methods of alcohol synthesis:
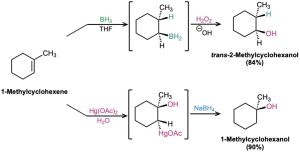
- Alcohols can be prepared by hydration of alkenes. Because the direct hydration of alkenes with aqueous acid is generally a poor reaction in the laboratory, two indirect methods are commonly used. Hydroboration–oxidation yields the syn, Anti-Markovnikov hydration product (Section 8.5), whereas oxymercuration– demercuration yields the Markovnikov hydration product (Section 8.4).
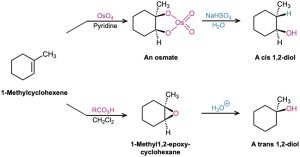
- 1,2-Diols can be prepared either by direct hydroxylation of an alkene with OsO4 followed by reduction with NaHSO3 or by acid-catalyzed hydrolysis of an epoxide (Section 8.7). The OsO4 reaction occurs with syn stereochemistry to give a cis diol, and epoxide opening occurs with anti stereochemistry to give a trans diol.
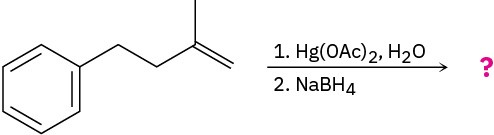
Problem 17-6
Predict the products of the following reactions:
(a)
![]()
(b)
(c)
![]()

