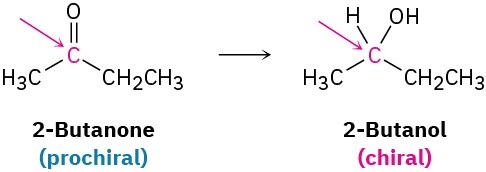
Closely related to the concept of chirality, and particularly important in biological chemistry, is the notion of prochirality. A molecule is said to be prochiral if it can be converted from achiral to chiral in a single chemical step. For instance, an unsymmetrical ketone like 2-butanone is prochiral because it can be converted to the chiral alcohol 2-butanol by the addition of hydrogen, as we’ll see in Section 17.4.
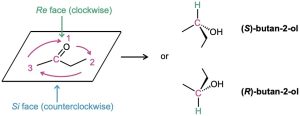
Which enantiomer of 2-butanol is produced depends on which face of the planar carbonyl group undergoes reaction. To distinguish between the possibilities, we use the stereochemical descriptors Re and Si. Rank the three groups attached to the trigonal, sp2– hybridized carbon, and imagine curved arrows from the highest to second-highest to third-highest ranked substituents. The face on which the arrows curve clockwise is designated the Re face (similar to R), and the face on which the arrows curve counterclockwise is designated the Si face (similar to S). In this example, addition of hydrogen from the Re face gives (S)-2-butane, and addition from the Si face gives (R)-2-butane.
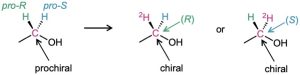
In addition to compounds with planar, sp2-hybridized atoms, compounds with tetrahedral, sp3-hybridized atoms can also be prochiral. An sp3-hybridized atom is said to be a prochiral center if, by changing one of its attached groups, it becomes a stereocenter. The −CH2OH carbon atom of ethanol, for instance, is a prochiral center because changing one of its attached −H atoms converts it into a stereocenter.
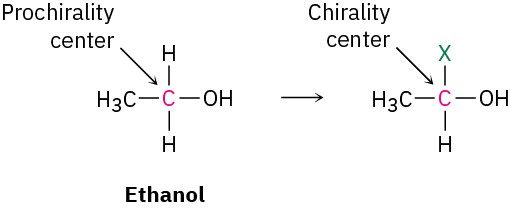
To distinguish between the two identical atoms (or groups of atoms) on a prochiral center, we imagine a change that will raise the ranking of one atom over the other without affecting its rank with respect to other attached groups. On the −CH2OH carbon of ethanol, for instance, we might imagine replacing one of the 1H atoms by 2H (deuterium). The newly introduced 2H atom ranks higher than the remaining 1H atom, but it remains lower than other groups attached to the carbon. Of the two identical atoms in the original compound, the atom whose replacement leads to an R stereocenter is said to be pro-R and the atom whose replacement leads to an S stereocenter is pro-S.
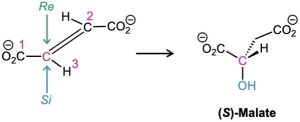
A large number of biological reactions involve prochiral compounds. One of the steps in the citric acid cycle by which food is metabolized, for instance, is the addition of H2O to fumarate to give malate. Addition of −OH occurs on the Si face of a fumarate carbon and gives (S)-malate as product.
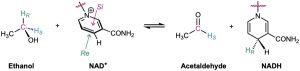
As another example, studies with deuterium-labeled substrates have shown that the reaction of ethanol with the coenzyme nicotinamide adenine dinucleotide (NAD+), catalyzed by yeast alcohol dehydrogenase, occurs with exclusive removal of the pro-R hydrogen from ethanol and with addition only to the Re face of NAD+.
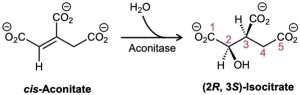
Determining the stereochemistry of reactions at prochiral centers is a powerful method for studying detailed mechanisms in biochemical reactions. As just one example, the conversion of citrate to cis-aconitate in the citric acid cycle has been shown to occur with loss of a pro-R hydrogen, implying that the OH and H groups leave from opposite sides of the molecule.

Problem 5-22
Identify the indicated hydrogens in the following molecules as pro-R or pro-S:
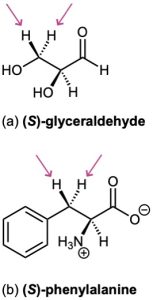
Problem 5-23
Identify the indicated faces of carbon atoms in the following molecules as Re or Si:
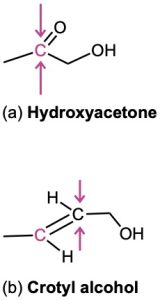
Problem 5-24
The lactic acid that builds up in tired muscles is formed from pyruvate. If the reaction occurs with addition of hydrogen to the Re face of pyruvate, what is the stereochemistry of the product?
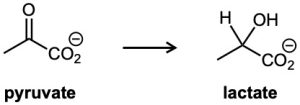
Problem 5-25
The aconitase-catalyzed addition of water to cis-aconitate in the citric acid cycle occurs with the following stereochemistry. Does the addition of the OH group occur on the Re or Si face of the substrate? What about the addition of the H? Do the H and OH groups add from the same side of the double bond or from opposite sides?