The reaction discussed in the previous section involves an addition to an achiral reactant and forms an optically inactive, racemic mixture of two enantiomeric products. What would happen, though, if we were to carry out the reaction on a single enantiomer of a chiral reactant? For example, what stereochemical result would be obtained from addition of H2O to a chiral alkene, such as (R)-4-methyl-1-hexene? The product of the reaction, 4-methyl-2-hexanol, has two chiral centers and so has four possible stereoisomers.
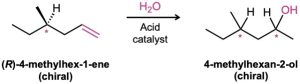
Let’s think about the two chiral centers separately. What about the configuration at C4, the methyl-bearing carbon atom? Since C4 has the R configuration in the starting material and this chiral center is unaffected by the reaction, its configuration is unchanged. Thus, the configuration at C4 in the product remains R (assuming that the relative rankings of the four attached groups are not changed by the reaction).
What about the configuration at C2, the newly formed chiral center? As shown in Figure 8.14, the stereochemistry at C2 is established by reaction of H2O with a carbocation intermediate in the usual manner. But this carbocation doesn’t have a plane of symmetry; it is chiral because of the chiral center at C4. Because the carbocation is chiral and has no plane of symmetry, it doesn’t react equally well from the top and bottom faces. One of the two faces is likely, for steric reasons, to be a bit more accessible than the other, leading to a mixture of R and S products in some ratio other than 50 : 50. Thus, two diastereomeric products, (2R,4R)-4-methyl-2-hexanol and (2S,4R)-4-methyl-2-hexanol, are formed in unequal amounts, and the mixture is optically active.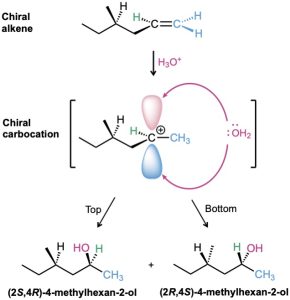
Figure 8.14 Stereochemistry of the acid-catalyzed addition of H2O to the chiral alkene, (R)-4-methyl-1-hexene. A mixture of diastereomeric 2R,4R and 2S,4R products is formed in unequal amounts because reaction of the chiral carbocation intermediate is not equally likely from top and bottom. The product mixture is optically active.
As a general rule, the formation of a new chiral center by a chiral reactant leads to unequal amounts of diastereomeric products. If the chiral reactant is optically active because only one enantiomer is used rather than a racemic mixture, then the products are also optically active.
Problem 8-20
What products are formed from acid-catalyzed hydration of racemic (±)-4-methyl-1-hexene? What can you say about the relative amounts of the products? Is the product mixture optically active?
Problem 8-21
What products are formed from hydration of 4-methylcyclopentene? What can you say about the relative amounts of the products?

