We’ve already seen several reactions of alcohols—their conversion into alkyl halides and tosylates in Section 10.5 and their dehydration to give alkenes in Section 8.1—albeit without mechanistic details. Let’s now look at those details.
Conversion of Alcohols into Alkyl Halides
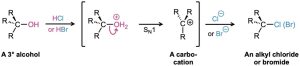
Tertiary alcohols react with either HCl or HBr by an SN1 mechanism through a carbocation intermediate. Primary and secondary alcohols are much more resistant to acid, however, and are best converted into halides by treatment with either SOCl2 or PBr3 through an SN2 mechanism.
The reaction of a tertiary alcohol with HX takes place by an SN1 mechanism when acid protonates the hydroxyl oxygen atom. Water is expelled to generate a carbocation, and the cation reacts with nucleophilic halide ion to give the alkyl halide product.
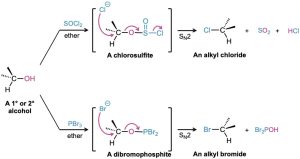
The reactions of primary and secondary alcohols with SOCl2 and PBr3 take place by SN2 mechanisms. Hydroxide ion itself is too poor a leaving group to be displaced by nucleophiles in SN2 reactions, but reaction of an alcohol with SOCl2 or PBr3 converts the alcohol into a chlorosulfite (–OSOCl) or a dibromophosphite (–OPBr2), respectively. These species are then readily expelled by nucleophilic substitution with chloride or bromide, respectively. Because this second step involves the backside attack of a halide nucleophile in a SN2 fashion, an inversion of configuration is observed. The resulting alkyl chloride or alkyl bromide products can then be used in a variety of transformations as reagents with a good leaving group.
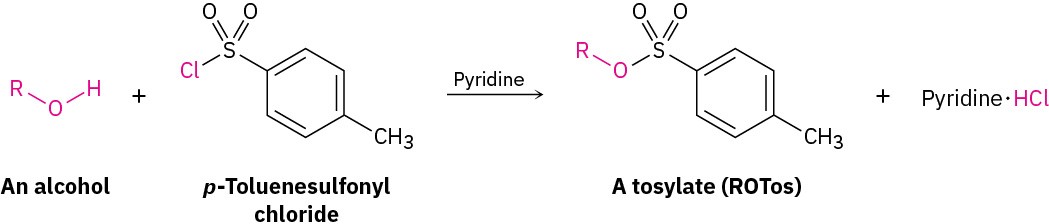
Conversion of Alcohols into Tosylates
Alcohols react with p-toluenesulfonyl chloride (tosyl chloride, p-TosCl) in pyridine solution to yield alkyl tosylates, ROTos (Section 11.1). Only the O–H bond of the alcohol is broken in this reaction; the C–O bond remains intact, so no change of configuration occurs if the oxygen is attached to a chiral center. The resultant alkyl tosylates behave much like alkyl halide leaving groups, undergoing both SN1 and SN2 substitution reactions.
One of the reasons to choose a tosylate in a SN2 reaction is stereochemical. Consider the following two sequences of reactions that both start with the R enantiomer of 2-octanol. The top sequence proceeds with two inversions of configuration—one to convert the alcohol to a halide and one to substitute the halide with ethoxide. This yields a product with the same stereochemistry (R) as the starting alcohol. However, the bottom sequence, which first converts the alcohol to a tosylate followed by nucleophilic substitution, proceeds with only one inversion and yields a product of opposite stereochemistry (S) relative to the starting alcohol.
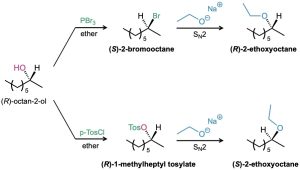
Figure 17.6 Stereochemical consequences of reactions of (R)-2-octanol.
Problem 17-12
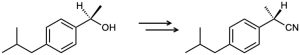
How would you carry out the following transformation, a step used in the commercial synthesis of (S)-ibuprofen?
Dehydration of Alcohols to Yield Alkenes
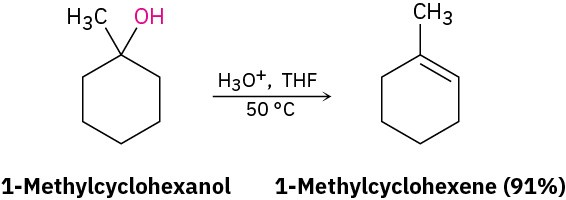
A third important reaction of alcohols, both in the laboratory and in biological pathways, is their dehydration to give alkenes. Because of the usefulness of the reaction, a number of methods have been devised for carrying out dehydrations. One method that works particularly well for tertiary alcohols is the acid-catalyzed reaction discussed in Section 8.1. For example, treatment of 1-methylcyclohexanol with warm, aqueous sulfuric acid in a solvent such as tetrahydrofuran results in loss of water and formation of 1- methylcyclohexene.
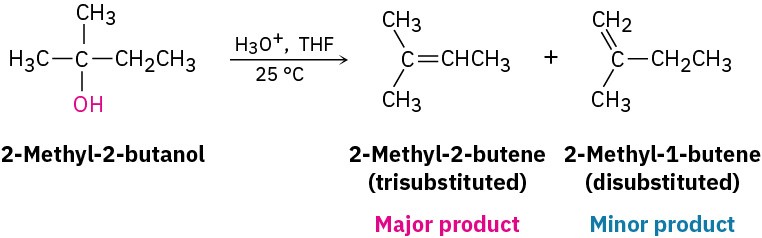
Acid-catalyzed dehydrations usually follow Zaitsev’s rule (Section 11.7) and yield the more stable alkene as the major product. Thus, 2-methyl-2-butanol gives primarily 2-methyl-2-butene (trisubstituted double bond) rather than 2-methyl-1-butene (disubstituted double bond).
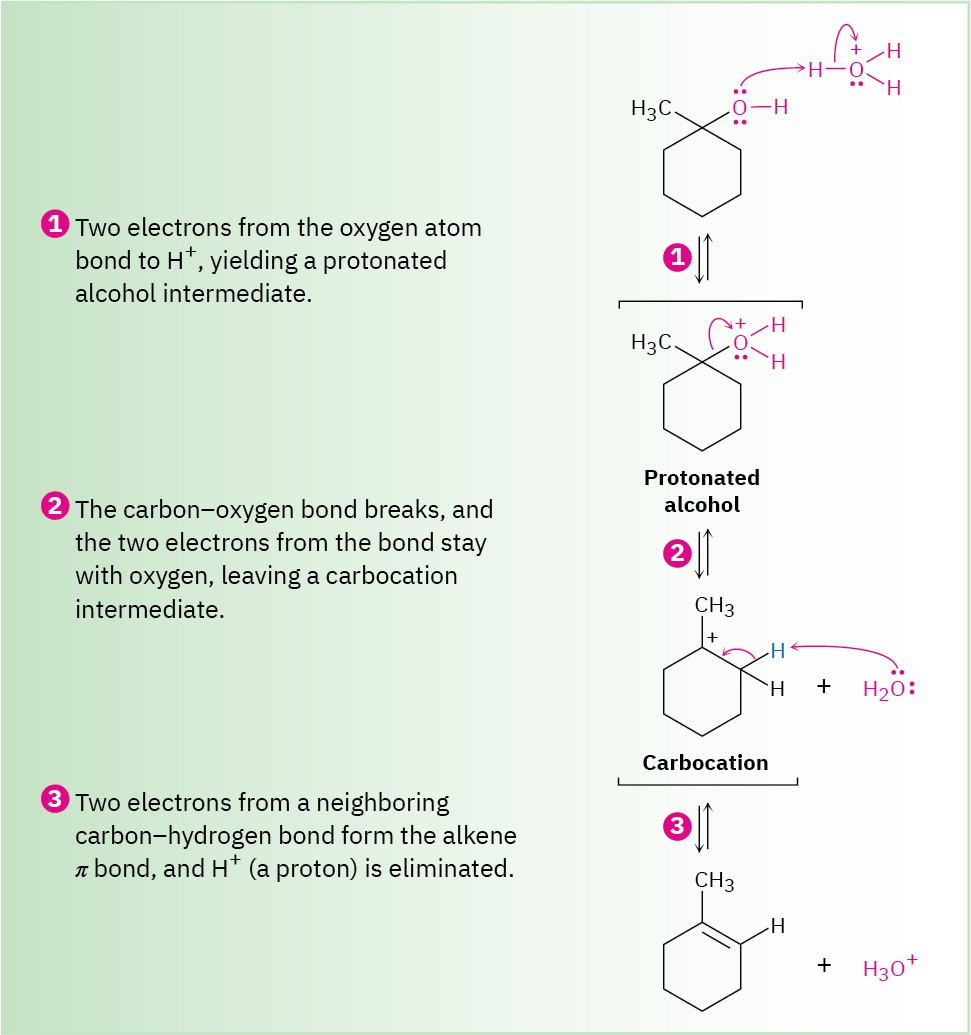
This reaction is an E1 process (Section 11.10) and occurs by the three-step mechanism shown in Figure 17.7. Protonation of the alcohol oxygen is followed by unimolecular loss of water to generate a carbocation intermediate and final loss of a proton from the neighboring carbon atom to complete the process. As with most E1 reactions, tertiary alcohols react fastest because they lead to stabilized, tertiary carbocation intermediates. Secondary alcohols can be made to react, but the conditions are severe (75% H2SO4, 100 °C) and fragile molecules risk decomposition.
Figure 17.7 MECHANISM
Mechanism for the acid-catalyzed dehydration of a tertiary alcohol to yield an alkene. The process is an E1 reaction and involves a carbocation intermediate.
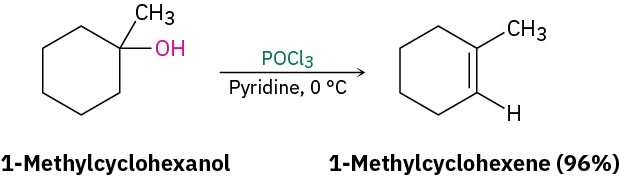
To circumvent the need for strong acid and allow the dehydration of secondary alcohols in a gentler way, reagents have been developed that are effective under mild, basic conditions. One such reagent, phosphorus oxychloride (POCl3) in the basic amine solvent pyridine, is often able to effect the dehydration of secondary and tertiary alcohols at 0 °C.
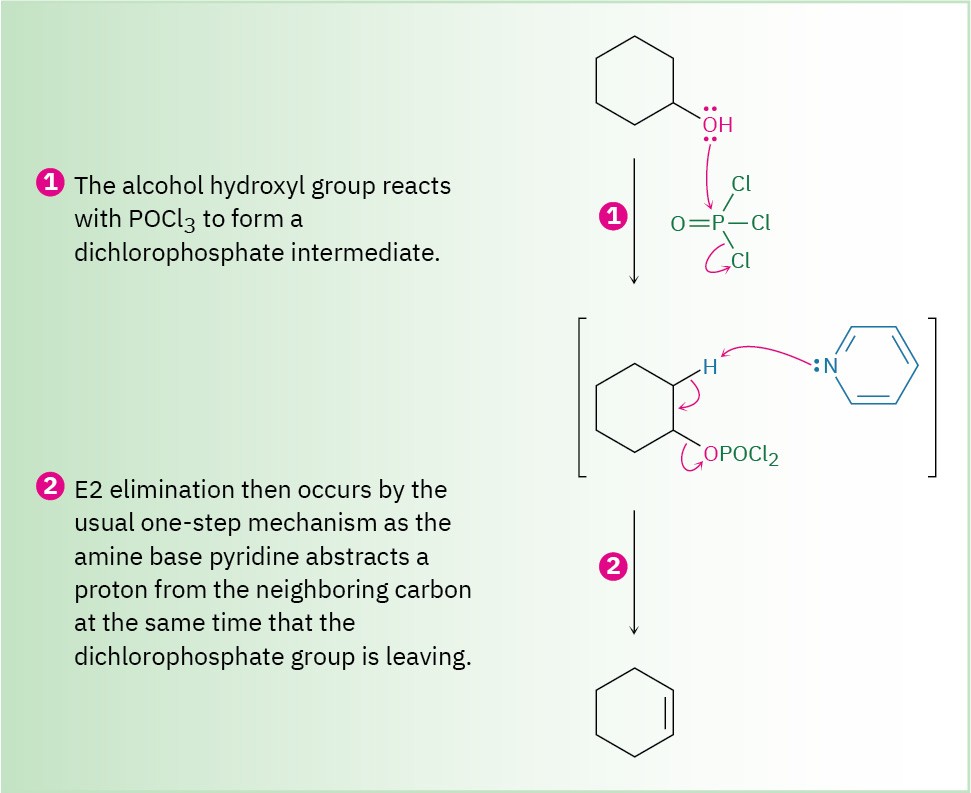
Alcohol dehydrations carried out with POCl3 in pyridine take place by an E2 mechanism, as shown in Figure 17.8. Because hydroxide ion is a poor leaving group (Section 11.3), direct E2 elimination of water from an alcohol does not occur. On reaction with POCl3, however, the alcohol group is converted into a dichlorophosphate (–OPOCl2), which is a good leaving group and is readily eliminated. Pyridine is both the reaction solvent and the base that removes a neighboring proton in the E2 elimination step.
Figure 17.8 Mechanism for the dehydration of secondary and tertiary alcohols by reaction with POCl3 in pyridine. The reaction is an E2 process.
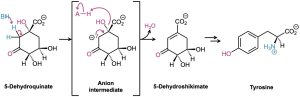
As noted in Section 11.11, biological dehydrations are also common and usually occur by an E1cB mechanism on a substrate in which the alcohol group is two carbons away from a carbonyl group. One example occurs in the biosynthesis of the aromatic amino acid tyrosine. A base (:B) first abstracts a proton from the carbon adjacent to the carbonyl group, and the anion intermediate then expels the alcohol group with simultaneous protonation by an acid (HA) to form water.
Problem 17-13
What product(s) would you expect from dehydration of the following alcohols with POCl3 in pyridine? Indicate the major product in each case.
(a)
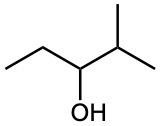
(b)
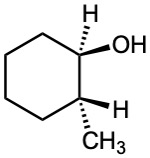
(c)

(d)
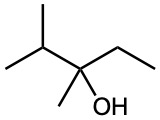
(e)
![]()
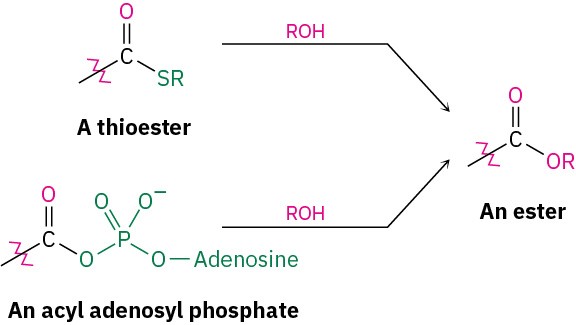
Conversion of Alcohols into Esters
Alcohols react with carboxylic acids to give esters, a reaction that is common in both the laboratory and living organisms. In the laboratory, the reaction can be carried out in a single step if a strong acid is used as catalyst. More frequently, though, the reactivity of the carboxylic acid is enhanced by first converting it into a carboxylic acid chloride, which then reacts with the alcohol.
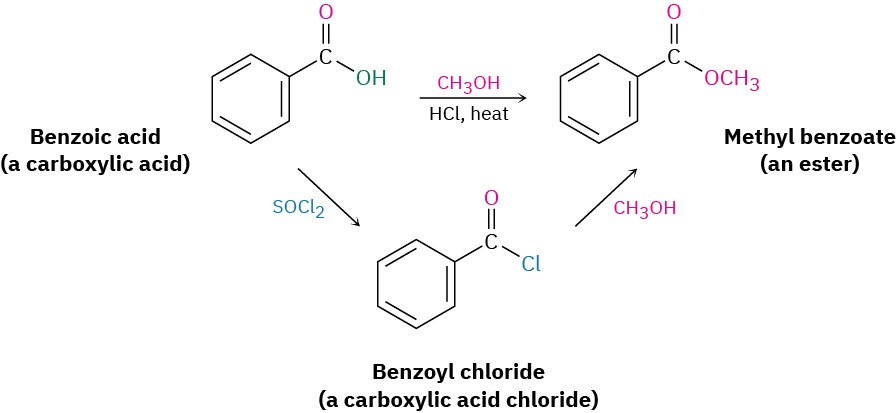
In living organisms, a similar process occurs, though a thioester or acyl adenosyl phosphate acts as substrate rather than a carboxylic acid chloride. We’ll look at the mechanisms of these reactions in Chapter 21.