You might recall from Section 1.9 that a carbon–carbon triple bond results from the interaction of two sp-hybridized carbon atoms. The two sp hybrid orbitals of carbon lie at an angle of 180° to each other along an axis perpendicular to the axes of the two unhybridized 2py and 2pz orbitals. When two sp-hybridized carbons approach each other, one sp–sp σ bond and two p–p π bonds are formed. The two remaining sp orbitals form bonds to other atoms at an angle of 180° from the carbon–carbon bond. Thus, acetylene is a linear molecule with H–C≡C bond angles of 180° (Figure 9.2). The length of the C≡C bond is 120 pm, and its strength is approximately 965 kJ/mol (231 kcal/mol), making it the shortest and strongest known carbon–carbon bond.
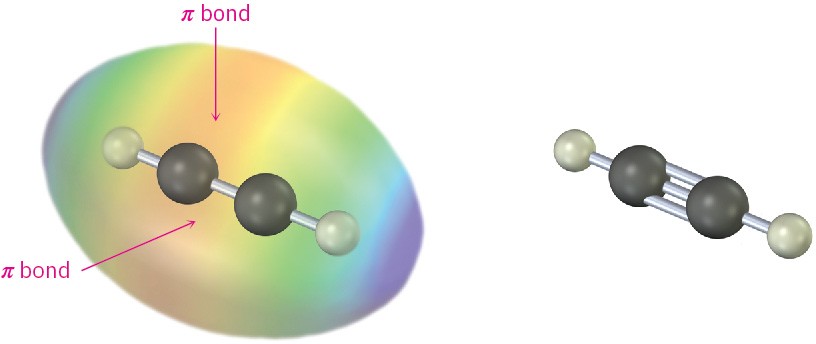
Figure 9.2 The structure of acetylene, H–C≡C–H. The H–C≡C bond angles are 180°, and the C≡C bond length is 120 pm. The electrostatic potential map shows that the π bonds create a negative belt around the molecule.
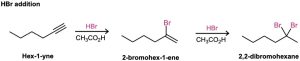
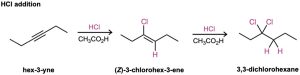
As a general rule, electrophiles undergo addition reactions with alkynes much as they do with alkenes. Take the reaction of alkynes with HX, for instance. The reaction often can be stopped with the addition of 1 equivalent of HX, but reaction with an excess of HX leads to a dihalide product. For example, reaction of 1-hexyne with 2 equivalents of HBr yields 2,2- dibromohexane. As the following examples indicate, the regiochemistry of addition follows Markovnikov’s rule, with the halogen adding to the more substituted side of the alkyne bond and hydrogen adding to the less substituted side. Trans stereochemistry of H and X normally, although not always, occurs in the product.
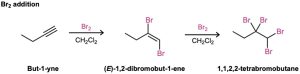
Diatomic bromine (Br2) and chlorine (Cl2) also add to alkynes to give addition products, and trans stereochemistry again results.
The mechanism of alkyne addition is similar but not identical to that of alkene addition. When an electrophile such as HBr adds to an alkene, the reaction takes place in two steps and involves an alkyl carbocation intermediate (Section 7.7 and Section 7.8). If HBr were to add by the same mechanism to an alkyne, an analogous vinylic carbocation would be formed as the intermediate.
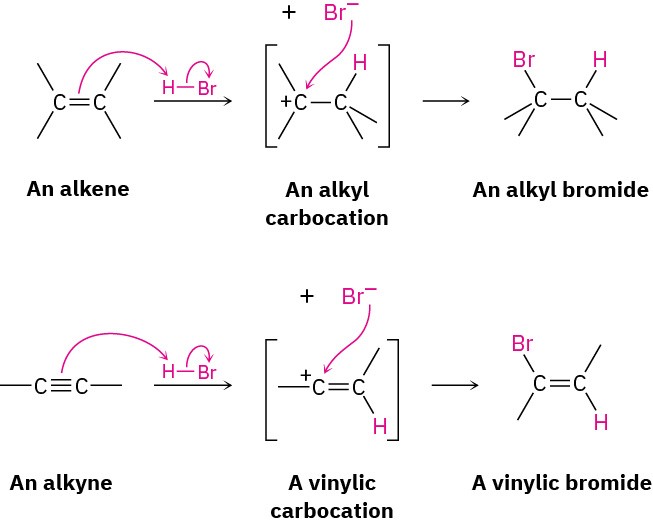
A vinylic carbocation has an sp-hybridized carbon and generally forms less readily than an alkyl carbocation (Figure 9.3). As a rule, a secondary vinylic carbocation forms about as readily as a primary alkyl carbocation, but a primary vinylic carbocation is so difficult to form that there is no clear evidence it even exists. Thus, many alkyne additions occur through more complex mechanistic pathways.
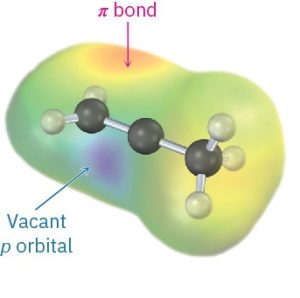
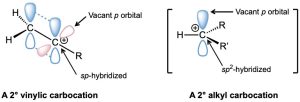
Figure 9.3 The structure of a secondary vinylic carbocation. The cationic carbon atom is sp-hybridized and has a vacant p orbital perpendicular to the plane of the π bond orbitals. Only one R group is attached to the positively charged carbon rather than two, as in a secondary alkyl carbocation. The electrostatic potential map shows that the most positive regions coincide with lobes of the vacant p orbital and are perpendicular to the most negative regions associated with the π bond.
Problem 9-3
What products would you expect from the following reactions?
(a)
![]()
(b)
![]()
(c)
![]()

