We commented earlier in this chapter that carboxylic acids are similar in some respects to both alcohols and ketones. Like alcohols, carboxylic acids can be deprotonated to give anions, which are good nucleophiles in SN2 reactions. Like ketones, carboxylic acids undergo addition of nucleophiles to the carbonyl group. However, carboxylic acids also undergo other reactions characteristic of neither alcohols nor ketones. Figure 20.3 shows some of the general reactions of carboxylic acids.
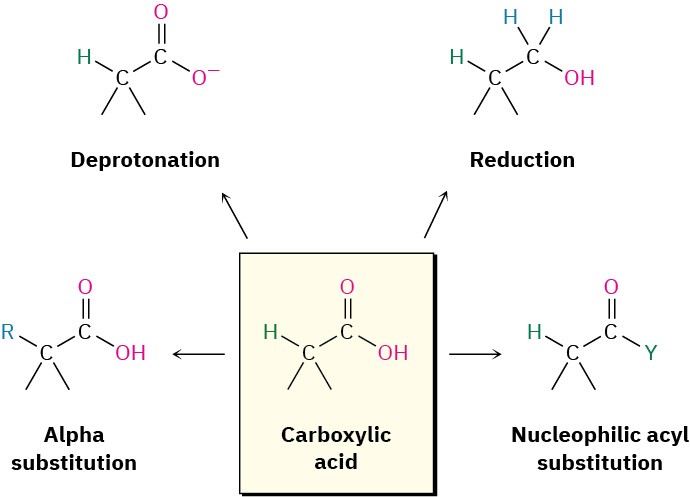
Figure 20.3 Some general reactions of carboxylic acids.
Reactions of carboxylic acids can be grouped into the four categories indicated in Figure 20.3. Of the four, we’ve already discussed the acidic behavior of carboxylic acids (Section 20.2-20.4), and we mentioned reduction by treatment with LiAlH4 in Section 17.4. The remaining two categories are examples of fundamental carbonyl-group reaction mechanisms—nucleophilic acyl substitution and α substitution—that will be discussed in detail in Chapters 21 and 22.
Problem 20-11
How might you prepare 2-phenylethanol from benzyl bromide? More than one step is needed.
![]()
Problem 20-12
How might you carry out the following transformation? More than one step is needed.
![]()

