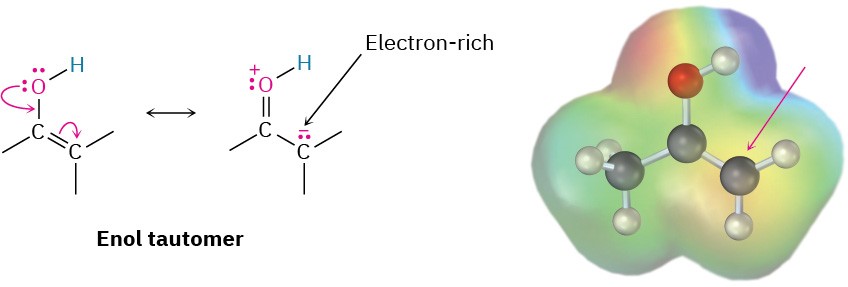
What kind of chemistry do enols have? Because their double bonds are electron-rich, enols behave as nucleophiles and react with electrophiles in much the same way that alkenes do. But because of resonance-electron donation of a lone pair of electrons on the neighboring oxygen, enols are more electron-rich and correspondingly more reactive than alkenes.
Notice in the following electrostatic potential map of ethenol (HC═CHOH) how there is a substantial electron density (yellow-red) on the α carbon.
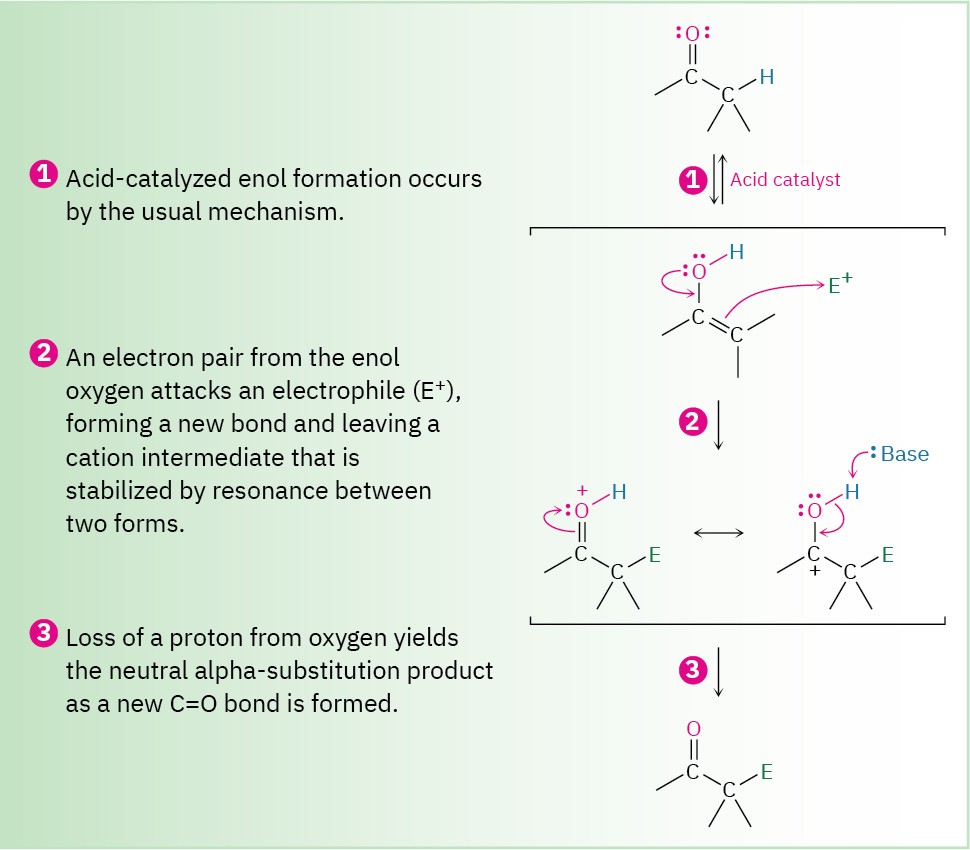
When an alkene reacts with an electrophile, E+, initial addition gives an intermediate cation and subsequent reaction with a nucleophile, such as a halide ion, yields an addition product (Section 7.7). When an enol reacts with an electrophile, however, only the initial addition step is the same (Figure 22.3). Instead of reacting with a nucleophile to give an addition product, the intermediate cation loses the –OH proton to give an α-substituted carbonyl compound.
Figure 22.3 General mechanism of a carbonyl α-substitution reaction.
In step 3, the initially formed cation loses H+ to regenerate a carbonyl compound.