Alkynes are reduced to alkanes by addition of H2 over a metal catalyst. The reaction occurs in two steps through an alkene intermediate, and measurements show that the first step in the reaction is more exothermic than the second.
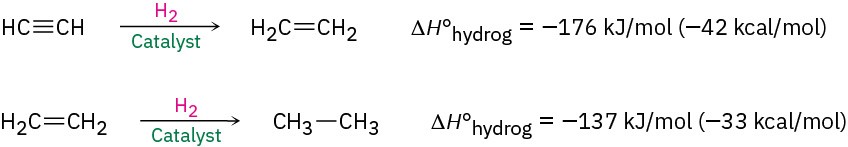
Complete reduction to the alkane occurs when palladium on carbon (Pd/C) is used as catalyst, but hydrogenation can be stopped at the alkene stage if the less active Lindlar catalyst is used. The Lindlar catalyst is a finely divided palladium metal that has been precipitated onto a calcium carbonate support and then deactivated by treatment with lead acetate and quinoline, an aromatic amine. The hydrogenation occurs with syn stereochemistry (Section 8.5), giving a cis alkene product.
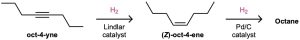
The alkyne hydrogenation reaction has been explored extensively by the Hoffmann– LaRoche pharmaceutical company, where it is used in the commercial synthesis of vitamin A. The cis isomer of vitamin A produced initially on hydrogenation is converted to the trans isomer by heating.
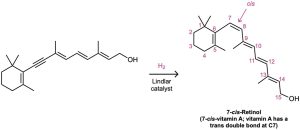
An alternative method for the conversion of an alkyne to an alkene uses sodium or lithium metal as the reducing agent in liquid ammonia as solvent. This method is complementary to the Lindlar reduction because it produces trans rather than cis alkenes. For example, 5-decyne gives trans-5-decene on treatment with lithium in liquid ammonia. The mechanism is explained below.
An electron first adds to the triple bond to yield an intermediate anion radical—a species that is both an anion (has a negative charge) and a radical (has an odd number of electrons). This anion radical is a strong base, able to remove H+ from ammonia to give a vinylic radical. Addition of a second electron to the vinylic radical gives a vinylic anion, which abstracts a second H+ from ammonia to give trans alkene product.
Figure 9.5 MECHANISM
Mechanism of the lithium/ammonia reduction of an alkyne to produce a trans alkene.
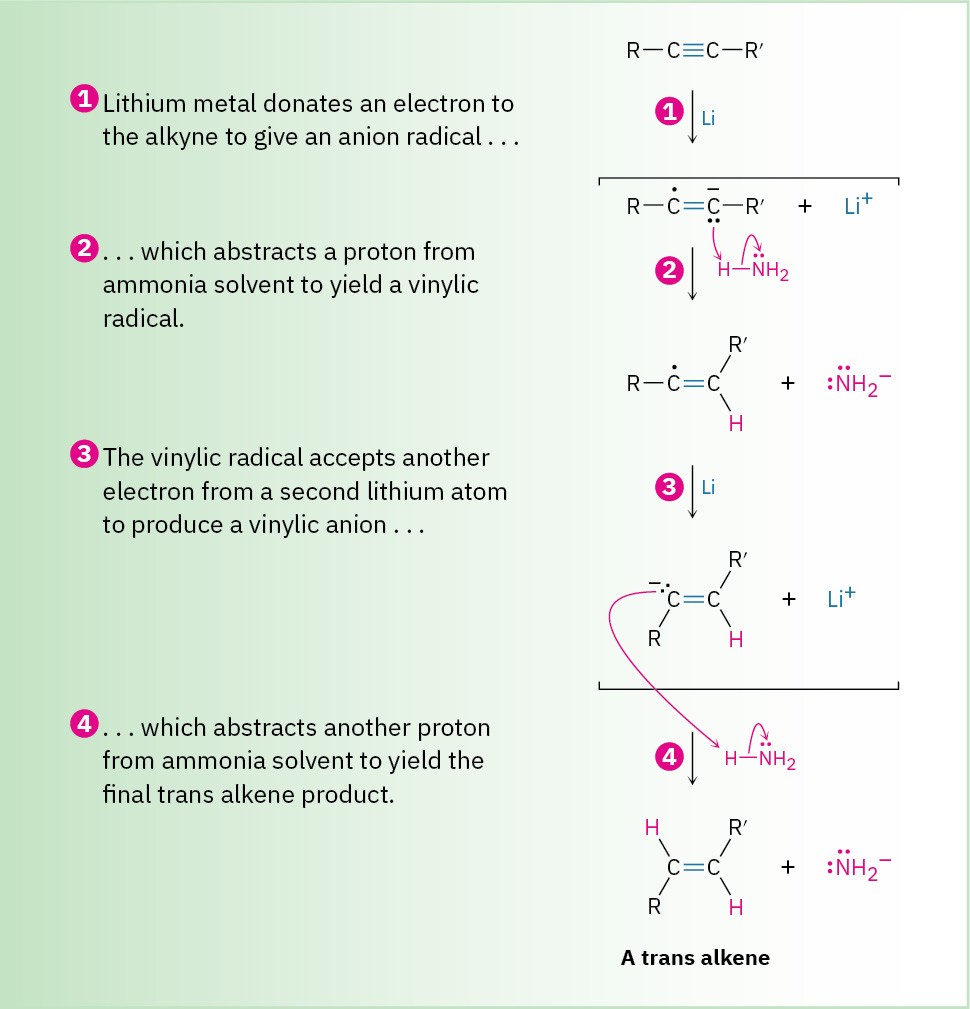
Trans stereochemistry of the alkene product is established during the second reduction step (3) when the less-hindered trans vinylic anion is formed from the vinylic radical.
Vinylic radicals undergo rapid cis–trans equilibration, but vinylic anions equilibrate much less rapidly. Thus, the more stable trans vinylic anion is formed rather than the less stable cis anion and is then protonated without equilibration.
Problem 9-8
Using any alkyne needed, how would you prepare the following alkenes?
(a) trans-2-Octene
(b) cis-3-Heptene
(c) Methyl-1-pentene

