Most substances can be represented unambiguously by the Lewis structures we’ve been using up to this point, but an interesting problem sometimes arises. Look at the acetate ion, for instance. When we draw a Lewis structure for acetate, we need to show a double bond to one oxygen and a single bond to the other. But which oxygen is which?
Should we draw a double bond to the “top” oxygen and a single bond to the “bottom” oxygen, or vice versa?

Although the two oxygen atoms in the acetate ion appear different in the Lewis structures, experiments show that they are equivalent. Both carbon–oxygen bonds, for instance, are 127 pm in length, midway between the length of a typical C–O single bond (135 pm) and a typical C=O double bond (120 pm). In other words, neither of the two structures for acetate is correct by itself. The true structure is an intermediate average between the two, in which both oxygen atoms share the negative charge.
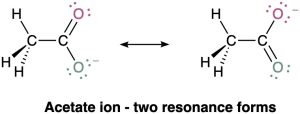
The two individual Lewis structures for acetate ion are called resonance forms or resonance contributors, and their special resonance relationship is indicated by the double-headed arrow between them. The only difference between the two resonance forms is the placement of the π and nonbonding valence electrons. The atoms themselves occupy exactly the same place in both resonance forms, the connections between atoms are the same, and the three-dimensional shapes of the resonance forms are the same.
Acetate does not convert back and forth between the two resonance forms, spending part of the time looking like one and part of the time looking like the other. Rather, acetate has a single unchanging structure that we say is a hybrid of the two individual resonance structure representations and has characteristics of both. The only “problem” here is that we can’t draw this true form accurately using our conventions of Lewis structure notation. The difficulty is with the representation of acetate via a Lewis structure, not with acetate itself.
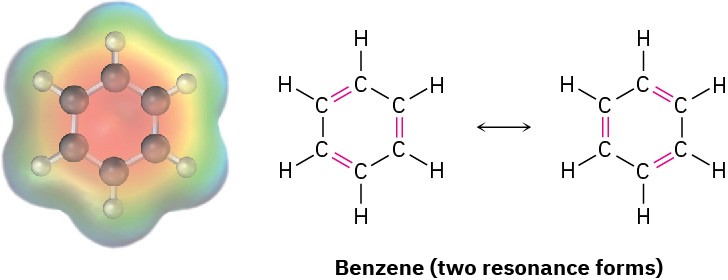
Resonance is a very useful concept that we’ll return to on numerous occasions throughout the rest of this book. We’ll see in Chapter 15, for instance, that the six carbon–carbon bonds in aromatic compounds, such as benzene, are equivalent and that benzene is best represented as a hybrid of two resonance forms. Although each individual resonance form seems to imply that benzene has alternating single and double bonds, neither form is correct by itself. The true benzene structure is a hybrid of the two individual forms, and all six carbon–carbon bonds are equivalent. This symmetrical distribution of electrons around the molecule is evident in an electrostatic potential map.