Structural drawings provide a visual representation of stereochemistry, but a written method for indicating the three-dimensional configuration, or arrangement, of substituents at a stereocenter is also needed. The method used a set of sequence rules to rank the four groups attached to the stereocenter and then looks at the handedness with which those groups are attached. Called the Cahn–Ingold–Prelog rules after the chemists who proposed them, the sequence rules are as follows:
RULE 1
Look at the four atoms directly attached to the stereocenter, and rank them according to atomic number. The atom with the highest atomic number has the highest ranking (first), and the atom with the lowest atomic number (usually hydrogen) has the lowest ranking (fourth). When different isotopes of the same element are compared, such as deuterium (2H) and protium (1H), the heavier isotope ranks higher than the lighter isotope. Thus, atoms commonly found in organic compounds have the following order.
![]()
RULE 2
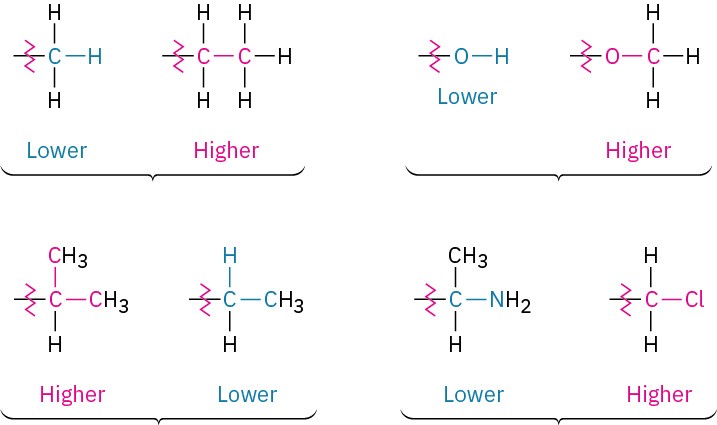
If a decision can’t be reached by ranking the first atoms in the substituent, look at the next set of atoms (moving away from the stereocenter) and continue until a priority can be assigned. A −CH2CH3 substituent and a −CH3 substituent are equivalent by rule 1 because both have carbon as the first atom. By rule 2, however, ethyl ranks higher than methyl because ethyl has a carbon as its highest second atom, while methyl has only hydrogen as its second atom. Look at the following pairs of examples to see how the rule works:
RULE 3
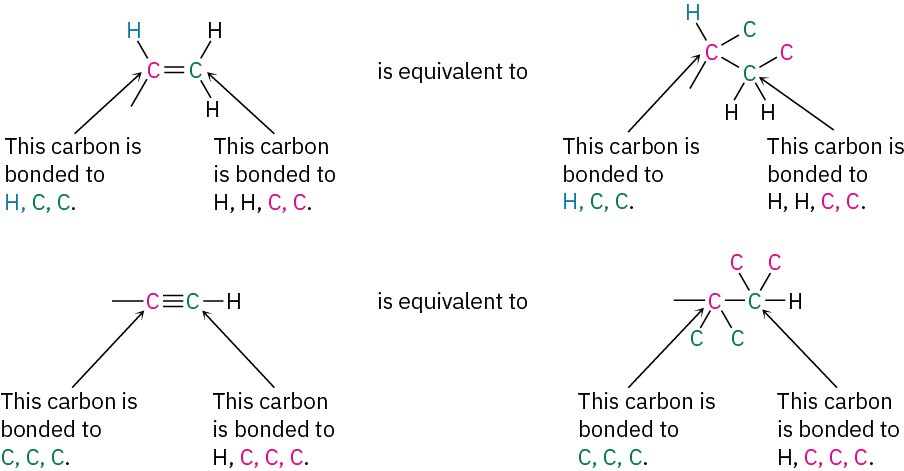
Multiple-bonded atoms are equivalent to the same number of single-bonded atoms. For example, an aldehyde substituent (–CH═O), which has a carbon atom doubly bonded to one oxygen, is equivalent to a substituent having a carbon atom singly bonded to two oxygens:
As further examples, the following pairs are equivalent:
Having ranked the four groups attached to a chiral carbon, we describe the stereochemical configuration around the carbon by orienting the molecule so that the group with the lowest ranking (4) points directly away from us. We then look at the three remaining substituents, which now appear to radiate toward us like the spokes on a steering wheel (Figure 5.8). If a curved arrow drawn from the highest to second-highest to third-highest ranked substituent (1 → 2 → 3) is clockwise, we say that the stereocenter has the R configuration (R from the Latin rectus, meaning “right”). If an arrow from 1 → 2 → 3 is counterclockwise, the stereocenter has the S configuration (S from the Latin sinister, meaning “left”).
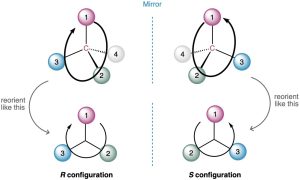
Figure 5.8 Assigning R and S configurations to stereocenters. When the molecule is oriented so that the lowest-ranked group (4) is toward the rear, the remaining three groups radiate toward the viewer like the spokes of a steering wheel. If the direction of travel 1 → 2 → 3 is clockwise (right turn), the center has the R configuration. If the direction of travel 1 → 2 → 3 is counterclockwise (left turn), the center is S.
Look at (−)-lactic acid in Figure 5.9 for an example of how to assign configuration. Sequence rule 1 says that −OH is ranked 1 and −H is ranked 4, but it doesn’t allow us to distinguish between −CH3 and −CO2H because both groups have carbon as their first atom. Sequence rule 2, however, says that −CO2H ranks higher than −CH3 because O (the highest second atom in −CO2H) outranks H (the highest second atom in −CH3). Now, turn the molecule so that the fourth-ranked group (−H) is oriented toward the rear, away from the observer.
Since a curved arrow from 1 (−OH) to 2 (−CO2H) to 3 (−CH3) is clockwise (right turn of the steering wheel), (−)-lactic acid has the R configuration. Applying the same procedure to (+)- lactic acid leads to the opposite assignment.
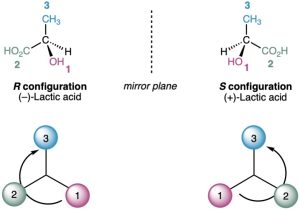
Figure 5.9 Assigning configuration to (a) (R)-(−)-lactic acid and (b) (S)-(+)-lactic acid.
Further examples are provided by naturally occurring (−)-glyceraldehyde and (+)-alanine, which both have the S configuration as shown in Figure 5.10. Note that the sign of optical rotation, (+) or (−), is not related to the R,S designation. (S)-Glyceraldehyde happens to be levorotatory (−), and (S)-alanine happens to be dextrorotatory (+). There is no simple correlation between R,S configuration and direction or magnitude of optical rotation.
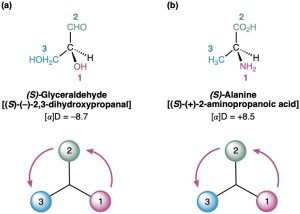
Figure 5.10 Assigning configuration to (a) (−)-glyceraldehyde. (b) (+)-alanine. Both happen to have the S configuration, although one is levorotatory and the other is dextrorotatory.
One additional point needs to be mentioned—the matter of absolute configuration. How do we know that the assignments of R and S configuration are correct in an absolute sense, rather than a relative, sense? Since there is no correlation between the R,S configuration and the direction or magnitude of optical rotation, how do we know that the R configuration belongs to the levorotatory enantiomer of lactic acid? This difficult question was finally solved in 1951, when an X-ray diffraction method was found for determining the absolute spatial arrangement of atoms in a molecule. Based on those results, we can say with certainty that the R,S conventions are correct.
Worked Example 5.3
Assigning Configuration to Stereocenters
Orient each of the following drawings so that the lowest-ranked group is toward the rear, and then assign R or S configuration to each:
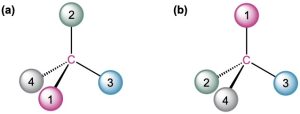
Strategy – It takes practice to be able to visualize and orient a stereocenter in three dimensions. You might start by indicating where the observer must be located—180° opposite the lowest-ranked group. Then imagine yourself in the position of the observer, and redraw what you would see.

Solution – In (a), you would be located in front of the page toward the top right of the molecule, and you would see group 2 to your left, group 3 to your right, and group 1 below you. This corresponds to an R configuration.
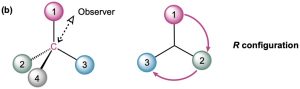
In (b), you would be located behind the page toward the top left of the molecule from your point of view, and you would see group 3 to your left, group 1 to your right, and group 2 below you. This also corresponds to an R configuration.
Worked Example 5.4
Drawing the Three-Dimensional Structure of a Specific Enantiomer
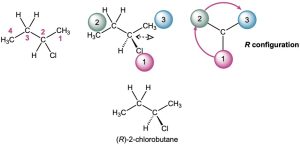
Draw a tetrahedral representation of (R)-2-chlorobutane.
Strategy – Begin by drawing the parent butane chain and placing the chlorine substituent at C2. Next, rank the four substituents bonded to the stereocenter: (1) −Cl, (2) −CH2CH3,(3) −CH3, (4) −H. To draw a tetrahedral representation of the molecule, we will place the lowest priority substituent on a hash and the chlorine atom on the wedge. Looking at the rotation starting at the priority 1 group and moving to group 2, then group 3, we see a clockwise rotation with the priority 4 group in the back (on a hash). This This is the R configuration. Had the priority 4 group been in the front (on a wedge) with the rotation being clockwise, this would be the S configuration.
Solution
Problem 5-7
Which member in each of the following sets ranks higher?
(a) −H or −Br
(b) −Cl or −Br
(c) −CH3 or −CH2CH3
(d) −NH2 or −OH
(e) −CH2OH or −CH3
(f) −CH2OH or −CH=O
Problem 5-8
Rank each of the following sets of substituents:
(a) −H, −OH, −CH2CH3, −CH2CH2OH
(b) −CO2H, −CO2CH3, −CH2OH, −OH
(c) −CN, −CH2NH2, −CH2NHCH3, −NH2
(d) −SH, −CH2SCH3, −CH3, −SSCH3
Problem 5-9
Draw the two chiral enantiomers of 2-methylbutane and assign the stereocenter in each as R or S.
Problem 5-10
Assign R or S configuration to the stereocenter in each of the following molecules:

Problem 5-11
Draw a tetrahedral representation of (S)-2-pentanol (2-hydroxypentane).
Problem 5-12
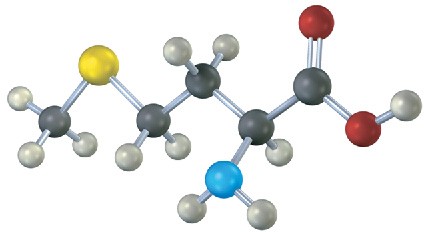
Assign R or S configuration to the stereocenter in the following molecular model of the amino acid methionine (blue = N, yellow = S):