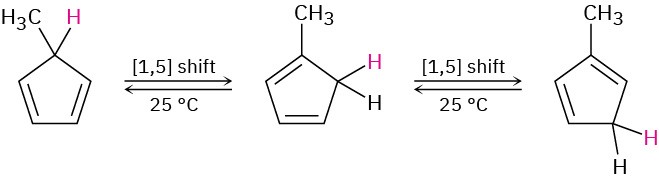
Because a [1,5] sigmatropic rearrangement involves three electron pairs (two π bonds and one σ bond), the orbital-symmetry rules in Table 30.3 predict a suprafacial reaction. In fact, the [1,5] suprafacial shift of a hydrogen atom across two double bonds of a π system is a commonly observed sigmatropic rearrangements. For example, 5-methyl-1,3-cyclopentadiene rapidly rearranges at room temperature to yield a mixture of 1-methyl-, 2- methyl-, and 5-methyl-isomers.
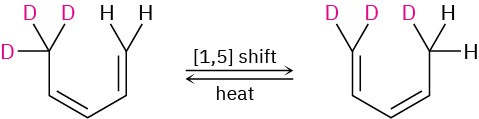
As another example, heating 5,5,5-trideuterio-(3Z)-1,3-pentadiene causes scrambling of deuterium between positions 1 and 5.
Both these [1,5] hydrogen shifts occur by a symmetry-allowed suprafacial pathway, as illustrated in Figure 30.13. In contrast with these thermal [1,5] sigmatropic hydrogen shifts, however, thermal [1,3] hydrogen shifts are unknown. If they were to occur, they would have to proceed by a strained antarafacial reaction pathway.
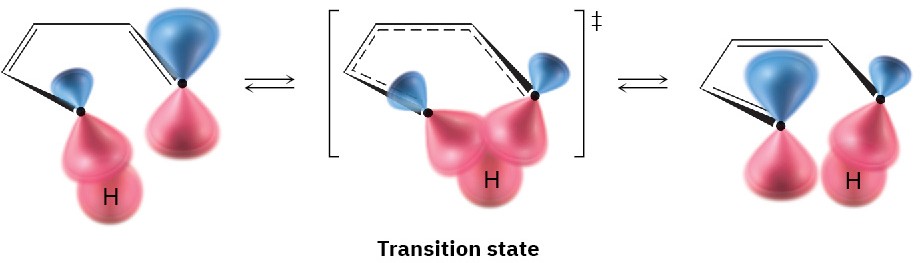
Figure 30.13 An orbital view of a suprafacial [1,5] hydrogen shift.
Two other important sigmatropic reactions are the Claisen rearrangement of either an allyl aryl ether (H𝟐C═CHCH𝟐─O─Ar) or an allyl vinyl (H𝟐C═CHCH𝟐─O─CH═CH𝟐) ether, and the Cope rearrangement of a 1,5 hexadiene to an isomeric 1,5-diene. These two rearrangements, along with the Diels–Alder reaction, are among the most generally useful pericyclic reactions for organic synthesis. Thousands of examples of all three are known.
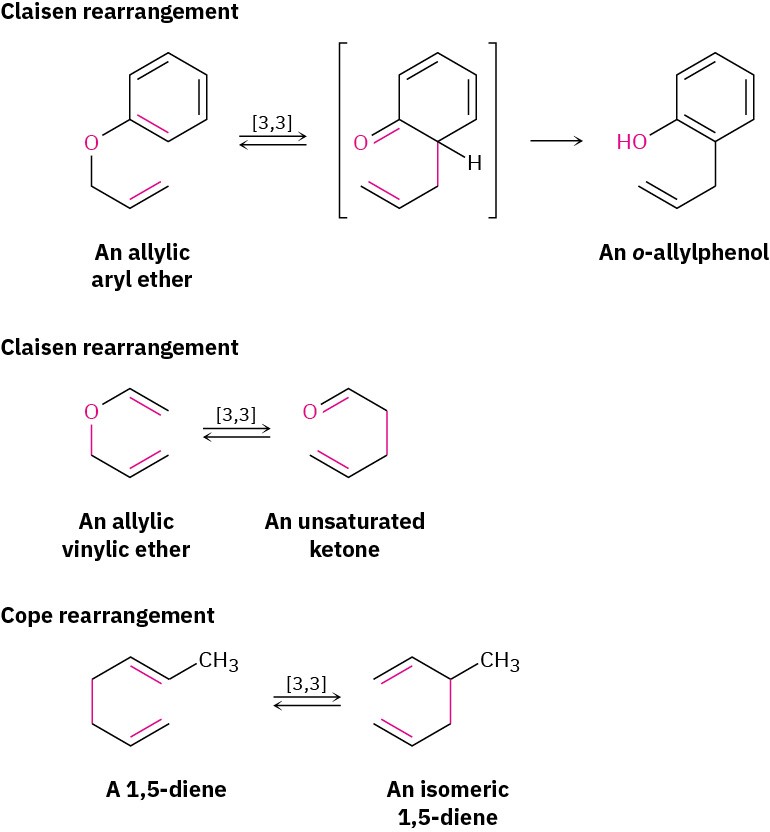
Like the Diels–Alder reaction discussed in Section 14.4 and Section 14.5, the Claisen rearrangement takes place in a single step through a pericyclic mechanism in which a reorganization of bonding electrons occurs in a six-membered, cyclic transition state. The 6-allyl-2,4-cyclohexadienone intermediate then isomerizes to o-allylphenol (Figure 30.14).
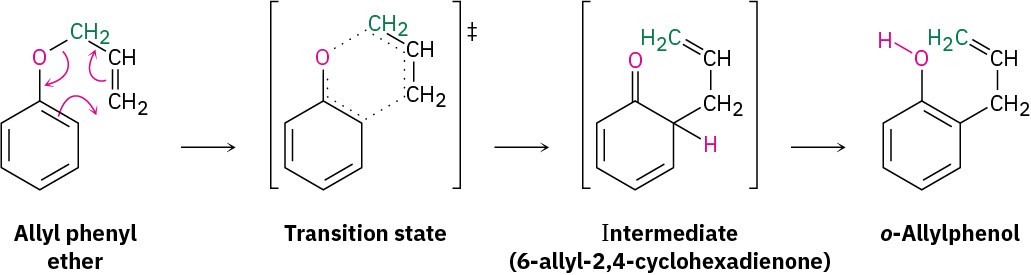
Figure 30.14 Mechanism of Claisen rearrangement. C─O bond-breaking and C─C bond-making occur simultaneously.
Evidence for this mechanism comes from the observation that the rearrangement takes place with transposition of the allyl group. That is, allyl phenyl ether containing a 14C label on the allyl ether carbon atom yields o-allylphenol in which the label is on the terminal vinylic carbon (green in Figure 30.14).
Problem 30-8
Draw a curved-arrow mechanism for the Cope arrangement just shown.
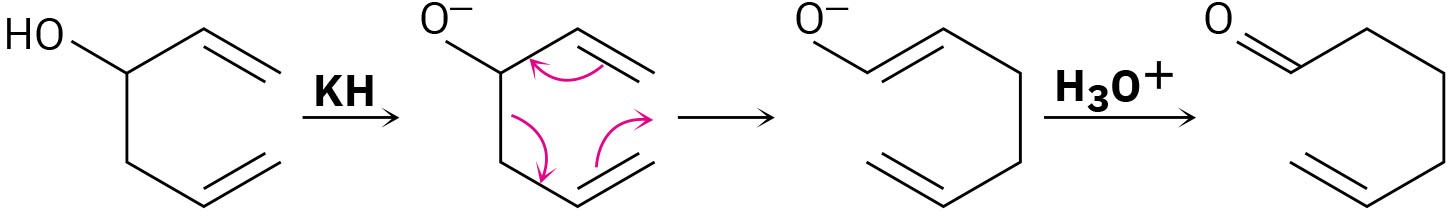
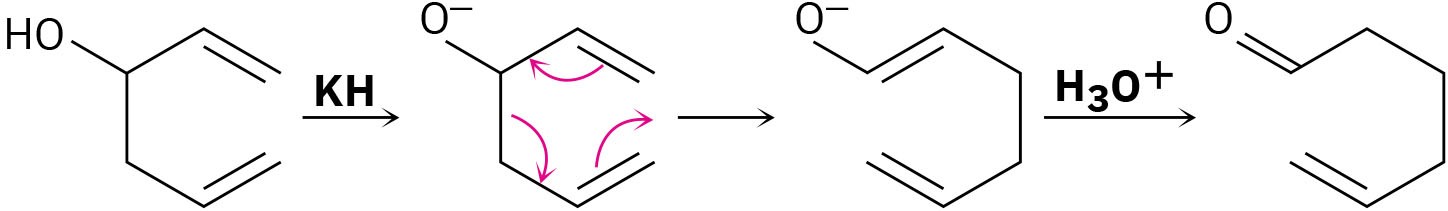
The Cope rearrangement that converts a 1,5-diene to an isomeric 1,5-diene is somewhat limited but a modification called the Oxy Cope Rearrangement is wider in scope. As shown in the following example, a 1-5 diene with an –OH next to the double bond can be converted into an oxy-anion by reaction with a strong base such as potassium hydride (KH). A Cope rearrangement then occurs, and reaction with aqueous acid gives an enol that tautomerizes to an aldehyde.
Although biological examples of pericyclic reactions are relatively rare, a much-studied example occurs in bacteria during biosynthesis of the essential amino acid phenylalanine. Phenylalanine arises from the precursor chorismate through a Claisen rearrangement to prephenate, followed by decarboxylation to phenylpyruvate and reductive amination (Figure 30.16). You might note that the reductive amination of phenylpyruvate is the exact reverse of the transamination process shown in Figure 29.17, by which amino acids are deaminated. In addition, the reductive amination of ketones is a standard method for preparing amines in the laboratory, as we saw in Section 24.6.
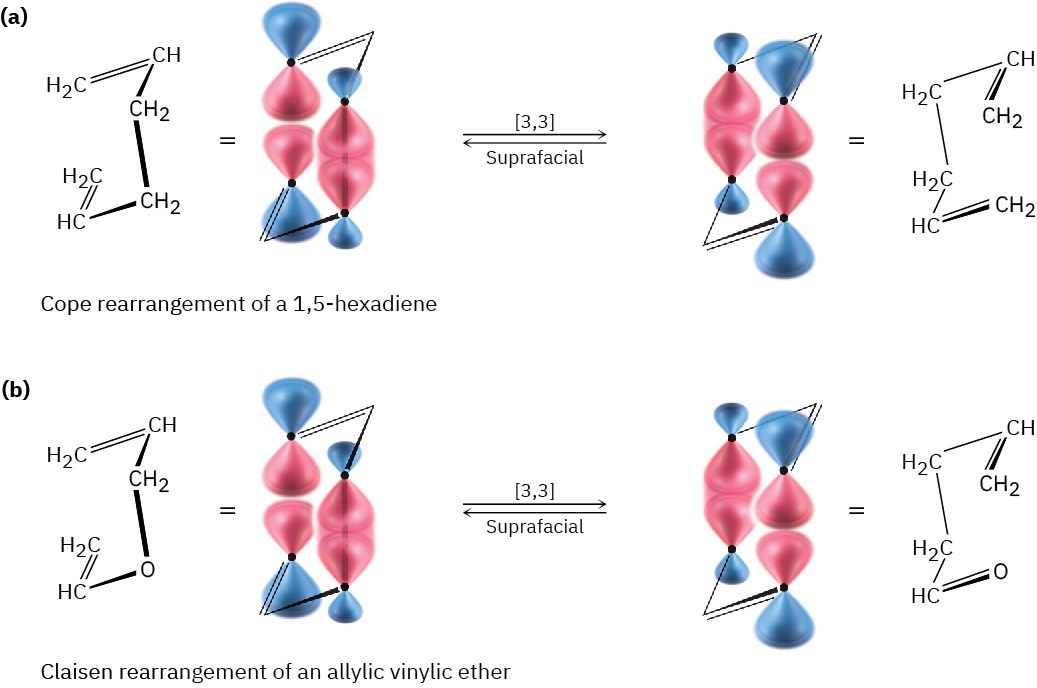
Figure 30.15 Suprafacial [3,3] (a) Cope and (b) Claisen rearrangements.
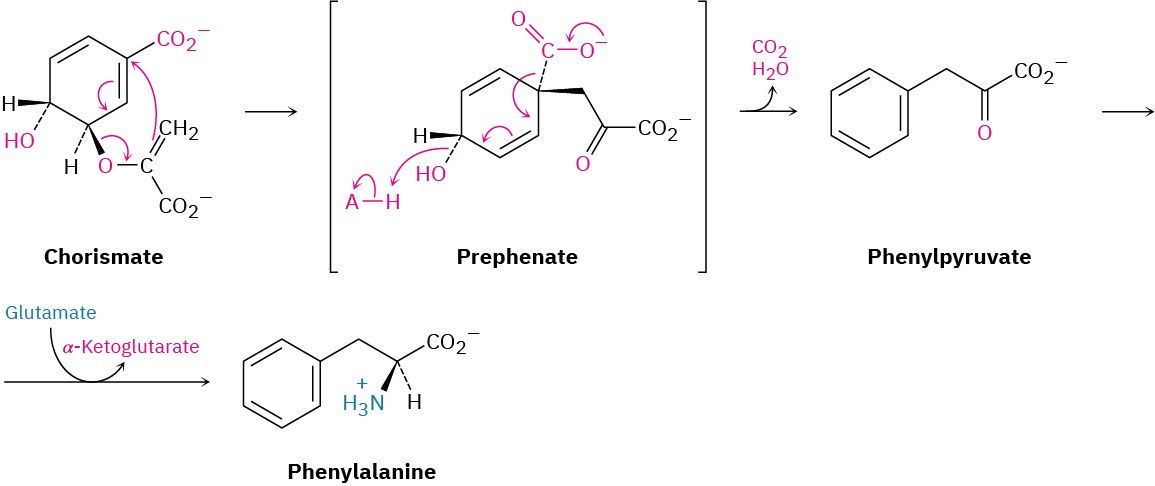
Figure 30.16 Pathway for the bacterial biosynthesis of phenylalanine from chorismate, involving a Claisen rearrangement.
Problem 30-9
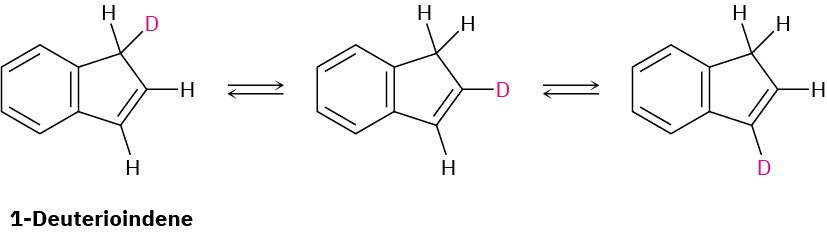
Propose a mechanism to account for the fact that heating 1-deuterioindene scrambles the isotope label to all three positions on the five-membered ring.
Problem 30-10
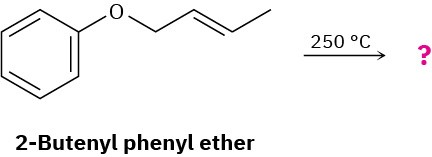
What product would you expect from Claisen rearrangement of 2-butenyl phenyl ether?
Problem 30-11
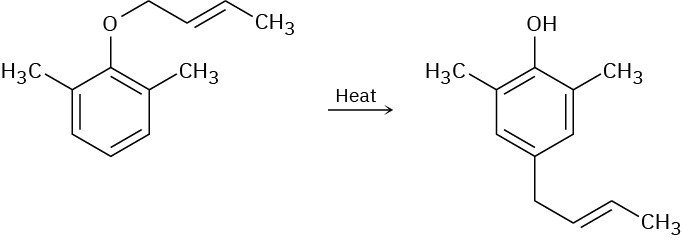
When a 2,6-disubstituted allyl phenyl ether is heated in an attempted Claisen rearrangement, migration occurs to give the p-allyl product as the result of two sequential pericyclic reactions. Explain.