Infrared Spectroscopy
Aldehydes and ketones show a strong C═O bond absorption in the IR region from 1660 to 1770 cm–1, as the spectra of benzaldehyde and cyclohexanone demonstrate (Figure 19.15). In addition, aldehydes show two characteristic C–H absorptions between 2700–2760 and 2800–2860 cm–1. These absorbances are important for distinguishing between aldehydes and ketones. The higher-frequency absorbance is sometimes obscured in cases where the compound has numerous saturated C–H groups, but the lower-frequency peak is almost always visible.
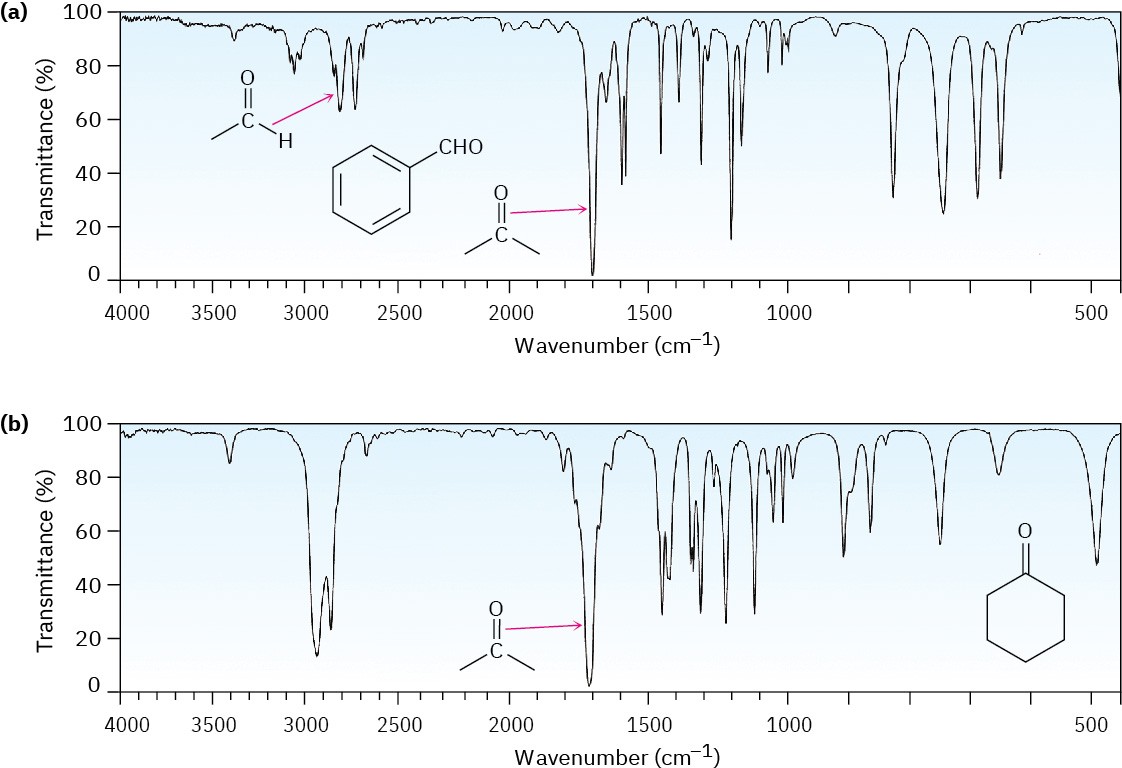
Figure 19.15 Infrared spectra of (a) benzaldehyde and (b) cyclohexanone.
The exact position of the C═O absorption is diagnostic of the nature of the carbonyl group. As the data in Table 19.2 indicate, saturated aldehydes usually show carbonyl absorptions near 1730 cm–1 in the IR spectrum, but conjugation of the aldehyde to an aromatic ring or a double bond lowers the absorption by 25 cm–1 to near 1705 cm–1. Saturated aliphatic ketones and cyclohexanones both absorb near 1715 cm–1, and conjugation with a double bond or an aromatic ring again lowers the absorption by 30 cm–1 to 1685–1690 cm–1. Angle strain in the carbonyl group, caused by reducing the ring size of cyclic ketones to four or five, raises the absorption position. Cyclohexanone has its C═O stretch absorbance at 1715 cm–1 while cyclopentanone’s carbonyl stretch is at 1750 cm–1 and cyclobutanone’s is at 1785 cm–1.
Table 19.2 Infrared Absorptions of Some Aldehydes and Ketones
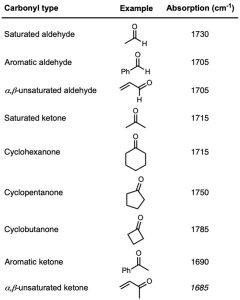
The values given in Table 19.2 are remarkably constant from one aldehyde or ketone to another. As a result, IR spectroscopy is a powerful tool for identifying the kind of a carbonyl group in a molecule of unknown structure. An unknown that shows an IR absorption at 1730 cm–1 is almost certainly an aldehyde rather than a ketone; an unknown that shows an IR absorption at 1750 cm–1 is almost certainly a cyclopentanone, and so on.
Problem 19-23
How might you use IR spectroscopy to determine whether reaction between 2-cyclohexenone and lithium dimethylcopper gives the direct addition product or the conjugate addition product?
Problem 19-24
Where would you expect each of the following compounds to absorb in the IR spectrum?
(a) 4-penten-2-one
(b) 3-penten-2-one
(c) 2,2-dimethylcyclopentanone
(d) m-chlorobenzaldehyde
(e) 3-cyclohexenone
(f) hexenal
Nuclear Magnetic Resonance Spectroscopy
Aldehyde protons (RCHO) absorb near 10 δ in the 1H NMR spectrum and are very distinctive because no other absorptions occur in this region. The aldehyde proton shows spin–spin coupling with protons on the neighboring carbon, with coupling constant J ≈ 3 Hz. Acetaldehyde, for example, shows a quartet at 9.79 δ for the aldehyde proton, indicating that there are three protons neighboring the –CHO group (Figure 19.16).
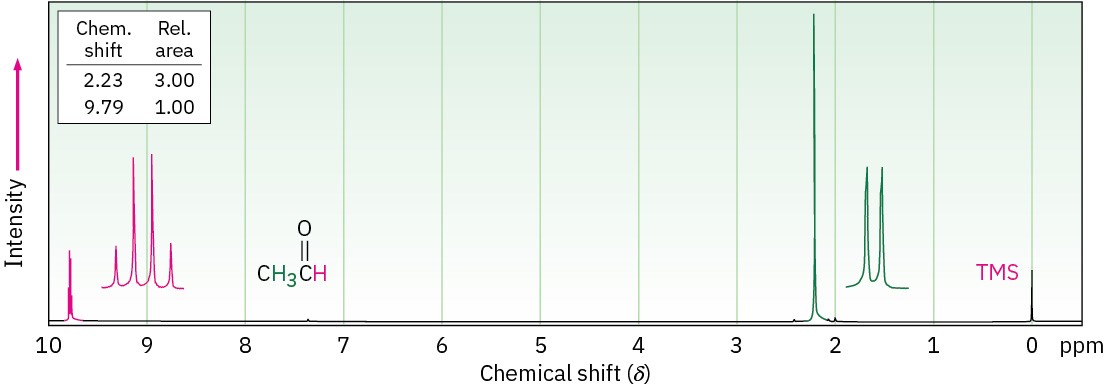
Figure 19.16 1H NMR spectrum of acetaldehyde. The absorption of the aldehyde proton appears at 9.79 δ and is split into a quartet.
Hydrogens on the carbon next to a carbonyl group are slightly deshielded and usually absorb near 2.0 to 2.3 δ. The acetaldehyde methyl group in Figure 19.16, for instance, absorbs at 2.20 δ. Methyl ketones are particularly distinctive because they always show a sharp three-proton singlet near 2.1 δ.
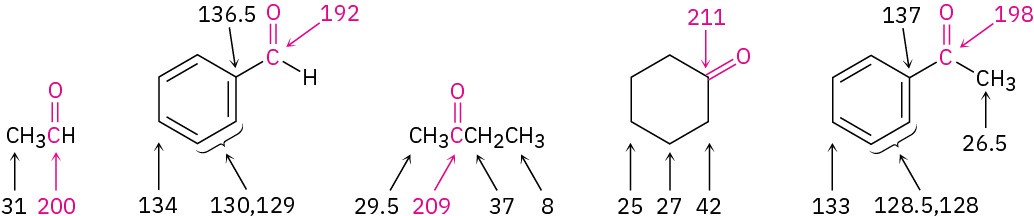
The carbonyl-group carbon atoms of aldehydes and ketones have characteristic 13C NMR resonances in the range 190 to 215 δ. Since no other kinds of carbons absorb in this range, the presence of an NMR absorption near 200 δ is clear evidence for a carbonyl group.
Saturated aldehyde or ketone carbons usually absorb in the region from 200 to 215 δ, while aromatic and α,β-unsaturated carbonyl carbons absorb in the 190 to 200 δ region.
Mass Spectrometry
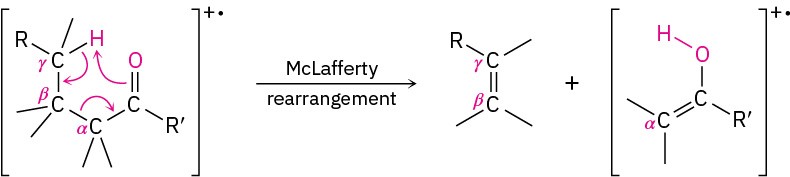
As discussed in Section 12.3, aliphatic aldehydes and ketones that have hydrogens on their gamma (γ) carbon atoms undergo a characteristic mass spectral cleavage called the McLafferty rearrangement. A hydrogen atom is transferred from the γ carbon to the carbonyl oxygen, the bond between the α and β carbons is broken, and a neutral alkene fragment is produced. The charge remains with the oxygen-containing fragment.
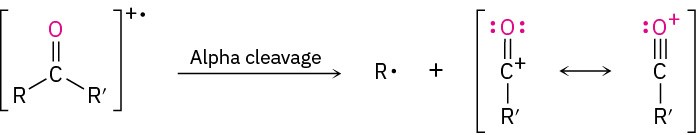
In addition to fragmentation by the McLafferty rearrangement, aldehydes and ketones also undergo cleavage of the bond between the carbonyl group and the α carbon, called an α cleavage. Alpha cleavage yields a neutral radical and a resonance-stabilized acyl cation.
Fragment ions from both McLafferty rearrangement and α cleavage are visible in the mass spectrum of 5-methyl-2-hexanone shown in Figure 19.17. McLafferty rearrangement with loss of 2-methylpropene yields a fragment with m/z = 58. Alpha cleavage occurs primarily at the more substituted side of the carbonyl group, leading to a [CH3CO]+ fragment with m/z = 43.
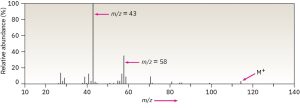
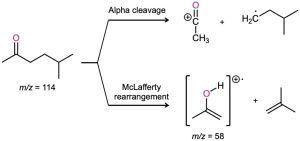
Figure 19.17 Mass spectrum and the related reactions of 5-methyl-2-hexanone. The peak at m/z = 58 is due to McLafferty rearrangement. The abundant peak at m/z = 43 is due to α cleavage at the more highly substituted side of the carbonyl group. Note that the peak due to the molecular ion is very small.
Problem 19-25
How might you use mass spectrometry to distinguish between the following pairs of isomers?
(a) methyl-2-hexanone and 4-methyl-2-hexanone
(b) 3-heptanone and 4-heptanone
(c) 2-methylpentanal and 3-methylpentanal
Problem 19-26
Describe the prominent IR absorptions and mass spectral peaks you would expect for the following compound: