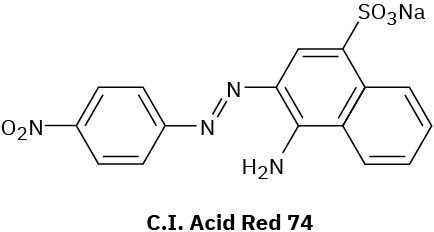
Infrared Spectroscopy
As many as four absorptions are sometimes observed in the 1450 to 1600 cm–1 region because of the complex molecular motions of the ring itself. Two bands, one at 1500 cm–1 and one at 1600 cm–1, are usually the most intense. In addition, aromatic compounds show weak absorptions in the 1660 to 2000 cm–1 region and strong absorptions in the 690 to 900 cm–1 range due to C–H out-of-plane bending. The exact position of both sets of absorptions is diagnostic of the substitution pattern of the aromatic ring (Figure 12.24 in Section 12.8).
|
Monosubstituted: |
690–710 cm–1 |
|
1,2,4-Trisubstituted: |
780–830 cm–1 |
|
|
730–770 cm–1 |
|
|
870–900 cm–1 |
|
o-Disubstituted: |
735–770 cm–1 |
|
1,2,3-Trisubstituted: |
670–720 cm–1 |
|
m-Disubstituted: |
690–710 cm–1 |
|
|
750–790 cm–1 |
|
|
810–850 cm–1 |
|
1,3,5-Trisubstituted: |
660–700 cm–1 |
|
p-Disubstituted: |
810–840 cm–1 |
|
|
830–900 cm–1 |
The IR spectrum of toluene in Figure 15.12 shows these characteristic absorptions.
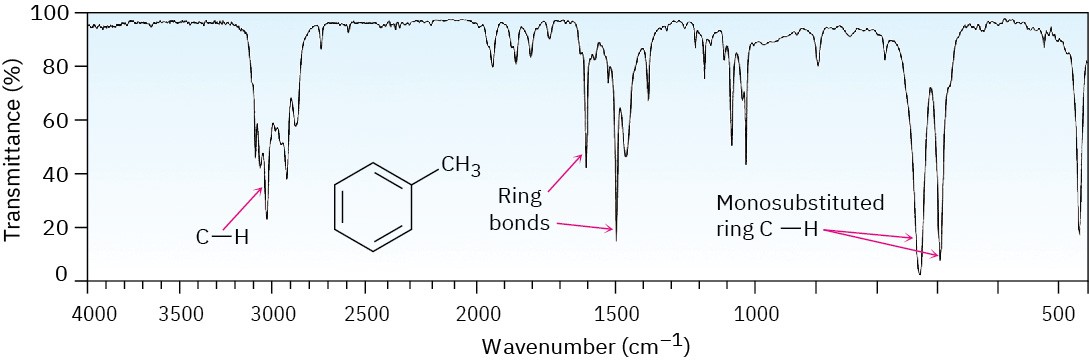
Figure 15.12 The infrared spectrum of toluene.
Ultraviolet Spectroscopy
Aromatic rings are detectable by ultraviolet spectroscopy because they contain a conjugated π electron system. In general, aromatic compounds show a series of bands, with a fairly intense absorption near 205 nm and a less intense absorption in the 255 to 275 nm range. The presence of these bands in the ultraviolet spectrum of a molecule is a sure indication of an aromatic ring. Figure 15.13 shows the ultraviolet spectrum of benzene.
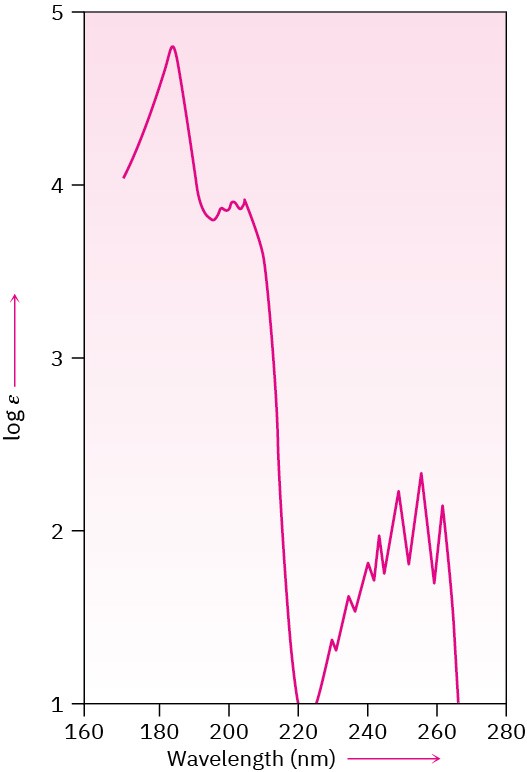
Figure 15.13 Ultraviolet spectrum of benzene. There are primary bands at 184 and 202 nm and secondary (fine-structure) bands at 255 nm.
Nuclear Magnetic Resonance Spectroscopy
Aromatic hydrogens are strongly deshielded by the ring and absorb between 6.5 and 8.0 δ. The spins of nonequivalent aromatic protons on substituted rings often couple with each other, giving rise to spin–spin splitting patterns that can identify the substitution of the ring.
Much of the difference in chemical shift between aromatic protons (6.5–8.0 δ) and vinylic protons (4.5–6.5 δ) is due to a property of aromatic rings called ring-current. When an aromatic ring is oriented perpendicular to a strong magnetic field, the electrons circulate around the ring, producing a small local magnetic field. This induced field opposes the applied field in the middle of the ring but reinforces the applied field outside the ring (Figure 15.14). Aromatic protons therefore experience an effective magnetic field greater than the applied field and come into resonance at a lower applied field.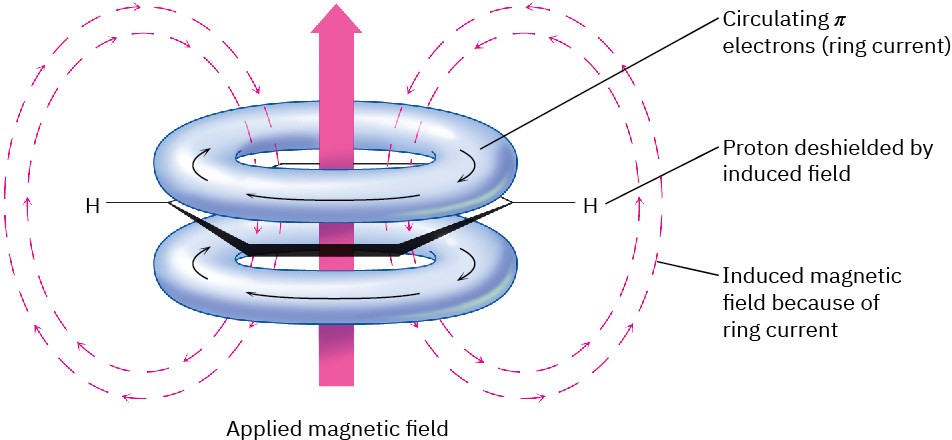
Figure 15.14The origin of aromatic ring-current. Aromatic protons are deshielded by the induced magnetic field caused by delocalized π electrons circulating around the aromatic ring.
Note that the aromatic ring-current produces different effects inside and outside the ring. If a ring were large enough to have both “inside” and “outside” protons, the protons on the outside would be deshielded and absorb at a field lower than normal but those on the inside would be shielded and absorb at a field higher than normal. This prediction has been strikingly confirmed by studies on [18]annulene, an 18-π-electron cyclic conjugated polyene that contains a Hückel number of electrons (4n + 2 = 18 when n = 4). The six inside protons of [18]annulene are strongly shielded by the aromatic ring-current and absorb at –3.0 δ (that is, 3.0 ppm upfield from TMS, off the normal chart), while the 12 outside protons are strongly deshielded and absorb in the typical aromatic region at 9.3 ppm downfield from TMS.
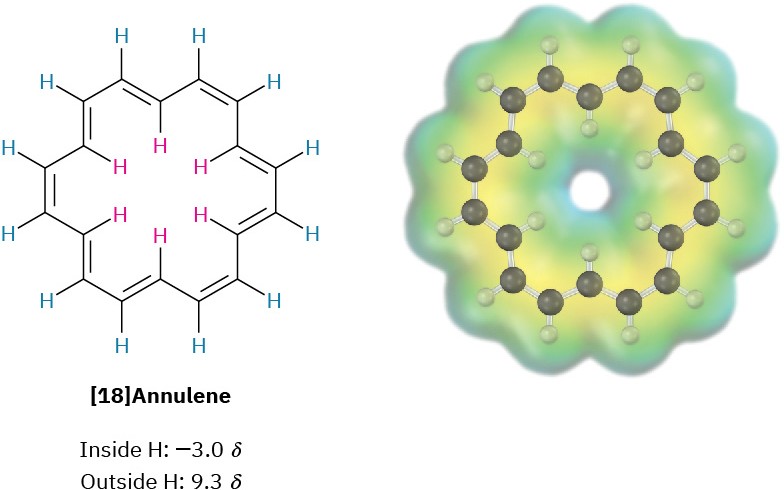 The presence of a ring-current is characteristic of all Hückel aromatic molecules and is a good test of aromaticity. For example, benzene, a six-π-electron aromatic molecule, absorbs at 7.37 δ because of its ring-current, but cyclooctatetraene, an eight-π-electron nonaromatic molecule, absorbs at 5.78 δ.
The presence of a ring-current is characteristic of all Hückel aromatic molecules and is a good test of aromaticity. For example, benzene, a six-π-electron aromatic molecule, absorbs at 7.37 δ because of its ring-current, but cyclooctatetraene, an eight-π-electron nonaromatic molecule, absorbs at 5.78 δ.
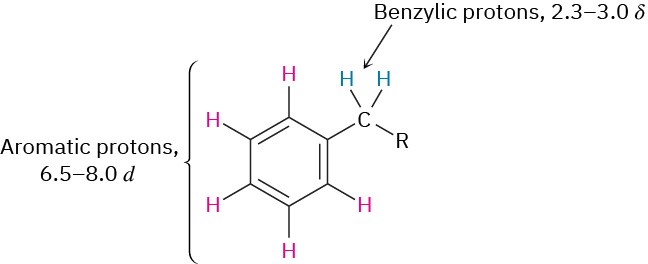
Hydrogens on carbon next to aromatic rings—benzylic hydrogens—also show distinctive absorptions in the NMR spectrum. Benzylic protons normally absorb downfield from other alkane protons in the region from 2.3 to 3.0 δ.
 The 1H NMR spectrum of p-bromotoluene, shown in Figure 15.15, displays many of the features just discussed. The aromatic protons appear as two doublets at 7.04 and 7.37 δ, and the benzylic methyl protons absorb as a sharp singlet at 2.26 δ. Integration of the spectrum shows the expected 2 : 2 : 3 ratio of peak areas.
The 1H NMR spectrum of p-bromotoluene, shown in Figure 15.15, displays many of the features just discussed. The aromatic protons appear as two doublets at 7.04 and 7.37 δ, and the benzylic methyl protons absorb as a sharp singlet at 2.26 δ. Integration of the spectrum shows the expected 2 : 2 : 3 ratio of peak areas.
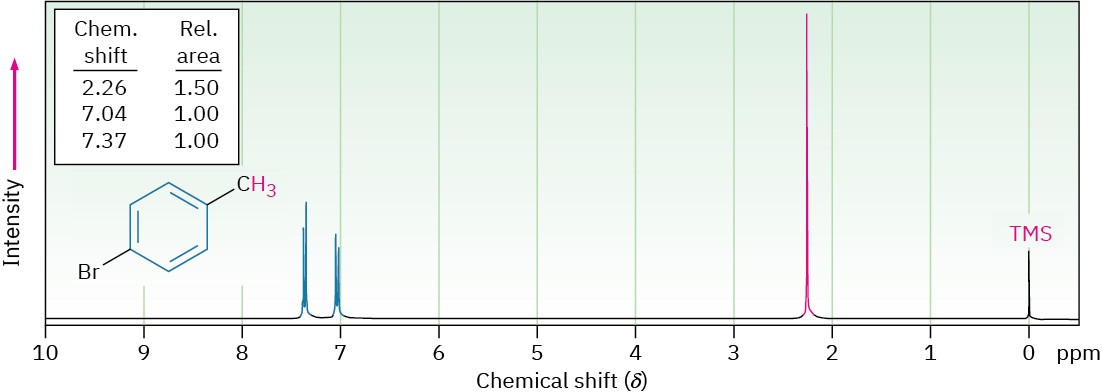 Figure 15.15 The 1H NMR spectrum of p-bromotoluene.
Figure 15.15 The 1H NMR spectrum of p-bromotoluene.
The carbon atoms in an aromatic ring typically absorb in the range 110 to 140 δ in the 13C NMR spectrum, as indicated by the examples in Figure 15.16. These resonances are easily distinguished from those of alkane carbons but occur in the same range as alkene carbons. Thus, the presence of 13C absorptions at 110 to 140 δ does not by itself establish the presence of an aromatic ring. Supporting evidence from infrared, ultraviolet, or 1H NMR spectra is needed.
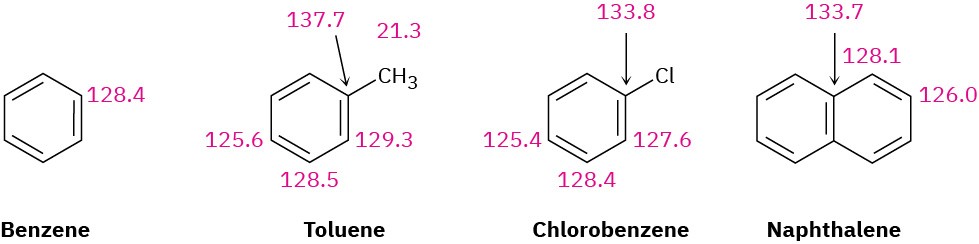 Figure 15.16 Some 13C NMR absorptions of aromatic compounds (δ units).
Figure 15.16 Some 13C NMR absorptions of aromatic compounds (δ units).
Problem 15-13
Give IUPAC names for the following substances (red = O, blue = N):
(a)
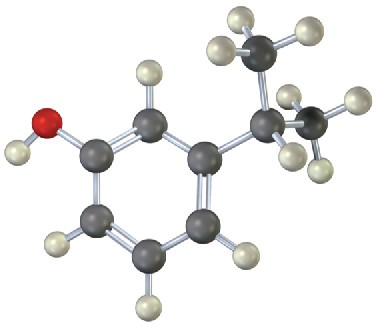
(b)
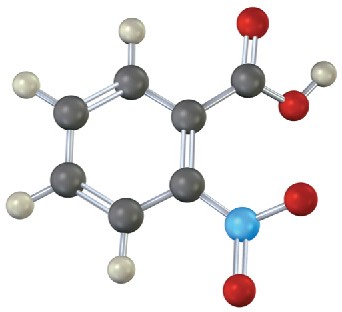
Problem 15-14
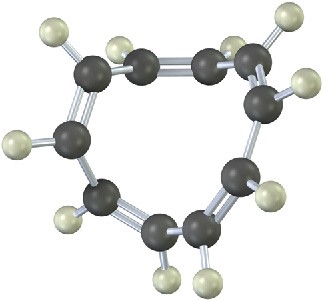
All-cis cyclodecapentaene is a stable molecule that shows a single absorption in its 1H NMR spectrum at 5.67 δ. Tell whether it is aromatic, and explain its NMR spectrum.
Problem 15-15
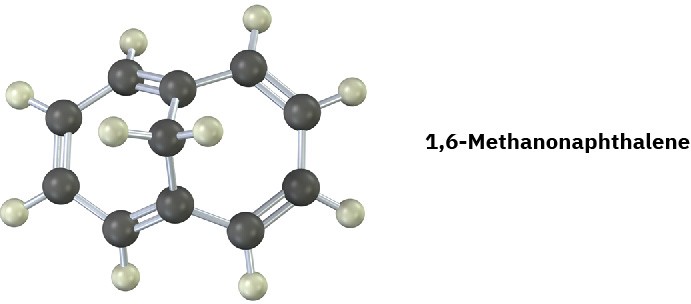
1,6-Methanonaphthalene has an interesting 1H NMR spectrum in which the eight hydrogens around the perimeter absorb at 6.9 to 7.3 δ, while the two CH2 protons absorb at –0.5 δ. Tell whether it is aromatic, and explain its NMR spectrum.
Problem 15-16
The following molecular model is that of a carbocation. Draw two resonance structures for the carbocation, indicating the positions of the double bonds.
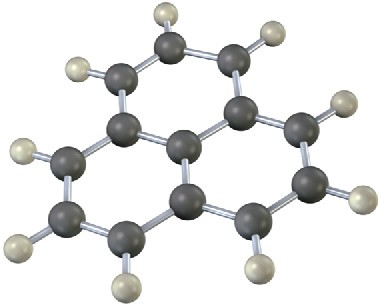
Problem 15-17
Azulene, an isomer of naphthalene, has a remarkably large dipole moment for a hydrocarbon (μ = 1.0 D). Explain, using resonance structures.
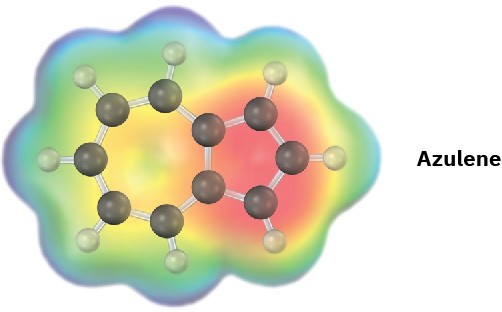
Naming Aromatic Compounds
Problem 15-18
Give IUPAC names for the following compounds:
(a)
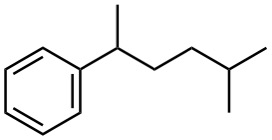
(b)
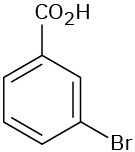
(c)
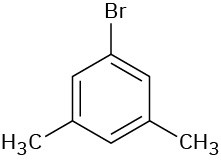
(d)
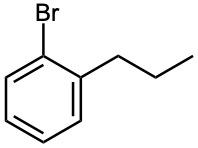
(e)
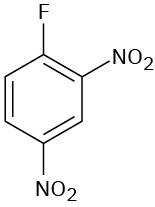
(f)
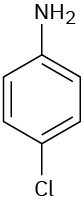
Problem 15-19
Draw structures corresponding to the following names:
(a) 3-Methyl-1,2-benzenediamine
(b) 1,3,5-Benzenetriol
(c) 3-Methyl-2-phenylhexane
(d) o-Aminobenzoic acid
(e) m-Bromophenol
(f) 2,4,6-Trinitrophenol (picric acid)
Problem 15-20
Draw and name all possible isomers of the following:
(a) Dinitrobenzene
(b) Bromodimethylbenzene
(c) Trinitrophenol
Problem 15-21
Draw and name all possible aromatic compounds with the formula C7H7Cl.
Problem 15-22
Draw and name all possible aromatic compounds with the formula C8H9Br. (There are 14.)
Structure of Aromatic Compounds
Problem 15-23
Propose structures for aromatic hydrocarbons that meet the following descriptions:
(a) C9H12; gives only one C9H11Br product on substitution of a hydrogen on the aromatic ring with bromine
(b) C10H14; gives only one C10H13Cl product on substitution of a hydrogen on the aromatic ring with chlorine
(c) C8H10; gives three C8H9Br products on substitution of a hydrogen on the aromatic ring with bromine
(d) C10H14; gives two C10H13Cl products on substitution of a hydrogen on the aromatic ring with chlorine
Problem 15-24
Look at the three resonance structures of naphthalene shown in Section 15.6, and account for the fact that not all carbon–carbon bonds have the same length. The C1–C2 bond is 136 pm long, whereas the C2–C3 bond is 139 pm long.
Problem 15-25
Anthracene has four resonance structures, one of which is shown. Draw the other three.
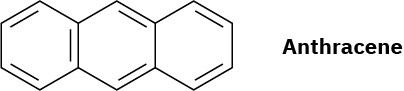
Problem 15-26
Phenanthrene has five resonance structures, one of which is shown. Draw the other four.
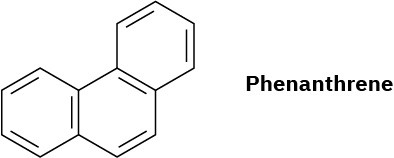
Problem 15-27
Look at the five resonance structures for phenanthrene (Problem 26), and predict which of its carbon–carbon bonds is shortest.
Problem 15-28
In 1932, A. A. Levine and A. G. Cole studied the ozonolysis of o-xylene and isolated three products: glyoxal, 2,3-butanedione, and pyruvaldehyde:
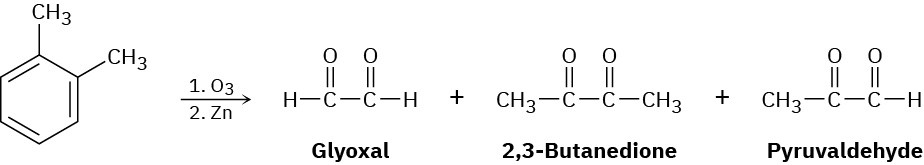
In what ratio would you expect the three products to be formed if o-xylene is a resonance hybrid of two structures? The actual ratio found was 3 parts glyoxal, 1 part 2,3-butanedione, and 2 parts pyruvaldehyde. What conclusions can you draw about the structure of o-xylene?
Aromaticity and Hückel’s Rule
Problem 15-29
3-Chlorocyclopropene, on treatment with AgBF4, gives a precipitate of AgCl and a stable solution of a product that shows a single 1H NMR absorption at 11.04 δ. What is a likely structure for the product, and what is its relation to Hückel’s rule?

Problem 15-30
Draw an energy diagram for the three molecular orbitals of the cyclopropenyl system (C3H3). How are these three molecular orbitals occupied in the cyclopropenyl anion, cation, and radical? Which of the three substances is aromatic according to Hückel’s rule?
Problem 15-31
Cyclopropanone is highly reactive because of its large amount of angle strain. Methylcyclopropenone, although even more strained than cyclopropanone, is nevertheless quite stable and can even be distilled. Explain, taking the polarity of the carbonyl group into account.
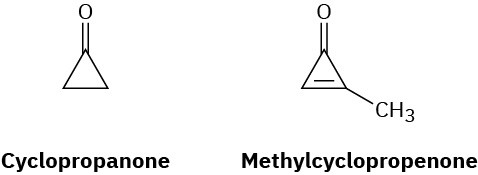
Problem 15-32
Cycloheptatrienone is stable, but cyclopentadienone is so reactive that it can’t be isolated. Explain, taking the polarity of the carbonyl group into account
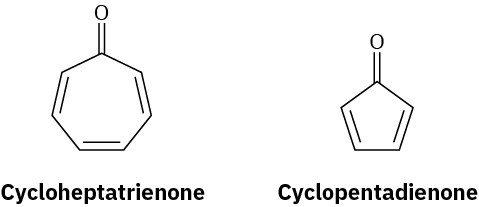
Problem 15-33
Which would you expect to be most stable, cyclononatetraenyl radical, cation, or anion? (Cyclononatetraene has a ring of nine carbons and four double bonds.)
Problem 15-34
How might you convert 1,3,5,7-cyclononatetraene to an aromatic substance? Problem 15-35
Calicene, like azulene (Problem 17), has an unusually large dipole moment for a hydrocarbon. Explain, using resonance structures.
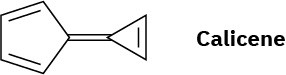
Problem 15-36
Pentalene is a most elusive molecule that has been isolated only at liquid-nitrogen temperature. The pentalene dianion, however, is well known and quite stable. Explain.
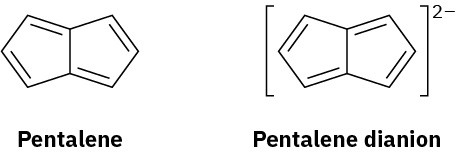
Problem 15-37
Indole is an aromatic heterocycle that has a benzene ring fused to a pyrrole ring. Draw an orbital picture of indole.
(a) How many π electrons does indole have?
(b) What is the electronic relationship of indole to naphthalene?
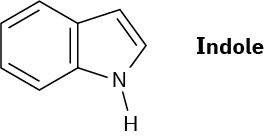
Problem 15-38
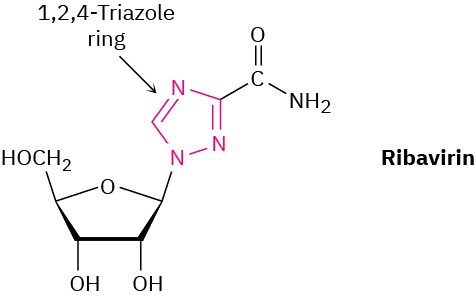
Ribavirin, an antiviral agent used against hepatitis C and viral pneumonia, contains a 1,2,4-triazole ring. Why is the ring aromatic?
Spectroscopy
Problem 15-39
Compound A, C8H10, yields three substitution products, C8H9Br, on reaction with Br2. Propose two possible structures for A. The 1H NMR spectrum of A shows a complex four- proton multiplet at 7.0 δ and a six-proton singlet at 2.30 δ. What is the structure of A?
Problem 15-40
What is the structure of a hydrocarbon that has M+ = 120 in its mass spectrum and has the following 1H NMR spectrum?
7.25 δ (5 H, broad singlet); 2.90 δ (1 H, septet, J = 7 Hz); 1.22 δ (6 H, doublet, J = 7 Hz)
Problem 15-41
Propose structures for compounds that fit the following descriptions:
(a) C10H14
1H NMR: 7.18 δ (4 H, broad singlet); 2.70 δ (4 H, quartet, J = 7 Hz); 1.20 δ (6 H, triplet, J = 7 Hz)
IR absorption at 745 cm–1
(b) C10H14
1H NMR: 7.0 δ (4 H, broad singlet); 2.85 δ (1 H, septet, J = 8 Hz); 2.28 δ (3 H, singlet); 1.20 δ (6 H, doublet, J = 8 Hz)
IR absorption at 825 cm–1
Problem 15-42
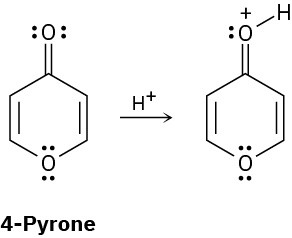
On reaction with acid, 4-pyrone is protonated on the carbonyl-group oxygen to give a stable cationic product. Using resonance structures and the Hückel 4n + 2 rule, explain why the protonated product is so stable.
Problem 15-43
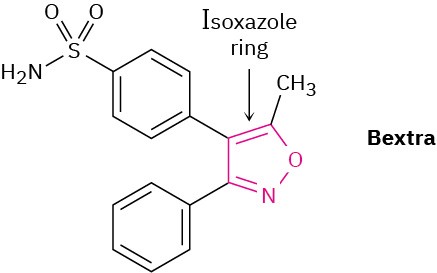
Bextra, a COX-2 inhibitor once used in the treatment of arthritis, contains an isoxazole ring. Why is the ring aromatic?
Problem 15-44
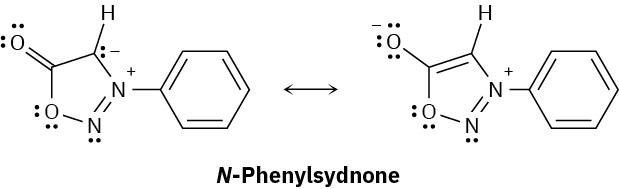
N-Phenylsydnone, so-named because it was first studied at the University of Sydney, Australia, behaves like a typical aromatic molecule. Explain, using the Hückel 4n + 2 rule.
Problem 15-45
Show the relative energy levels of the seven π molecular orbitals of the cycloheptatrienyl system. Tell which of the seven orbitals are filled in the cation, radical, and anion, and account for the aromaticity of the cycloheptatrienyl cation.
Problem 15-46
1-Phenyl-2-butene has an ultraviolet absorption at λmax = 208 nm (ε = 8000). On treatment with a small amount of strong acid, isomerization occurs and a new substance with λmax = 250 nm (ε = 15,800) is formed. Propose a structure for this isomer, and suggest a mechanism for its formation.
Problem 15-47
Propose structures for aromatic compounds that have the following 1H NMR spectra:
(a) C8H9Br
IR absorption at 820 cm–1
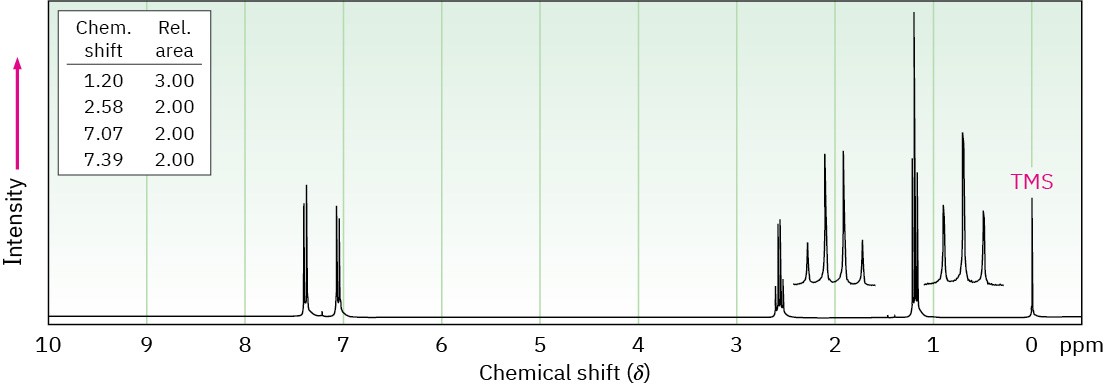
(b) C9H12
IR absorption at 750 cm–1
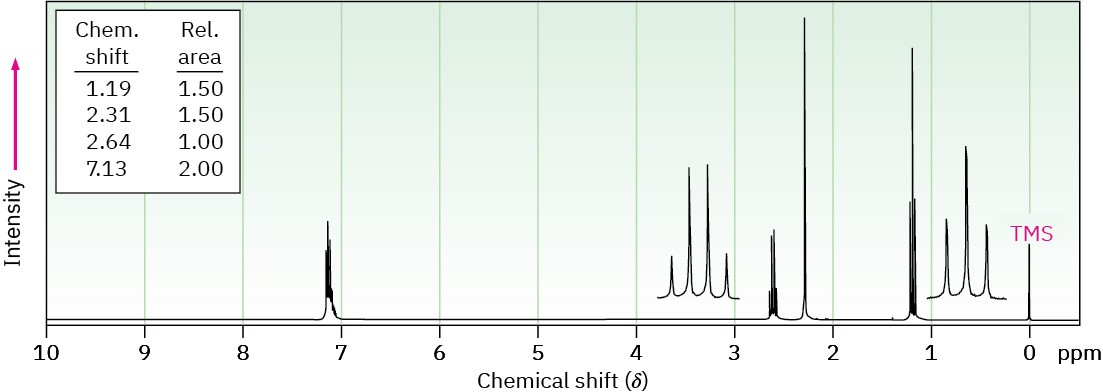
(c) C11H16
IR absorption at 820 cm–1
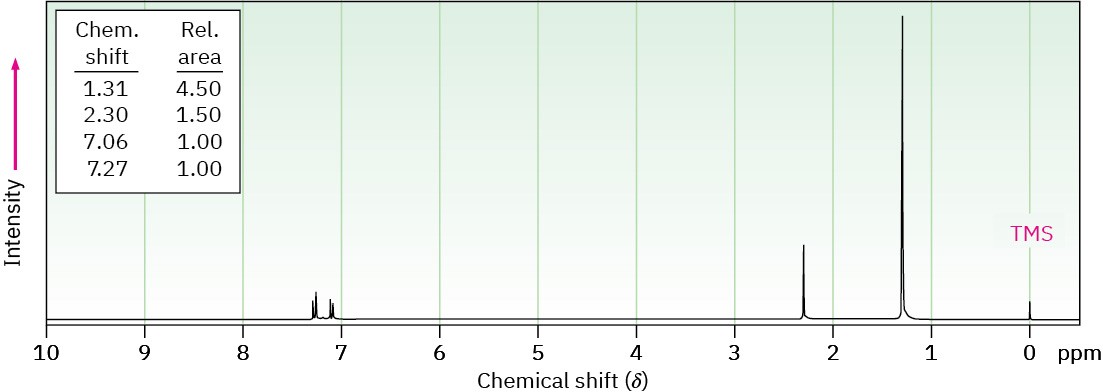
Problem 15-48
Propose a structure for a molecule C14H12 that has the following 1H NMR spectrum and has IR absorptions at 700, 740, and 890 cm–1:
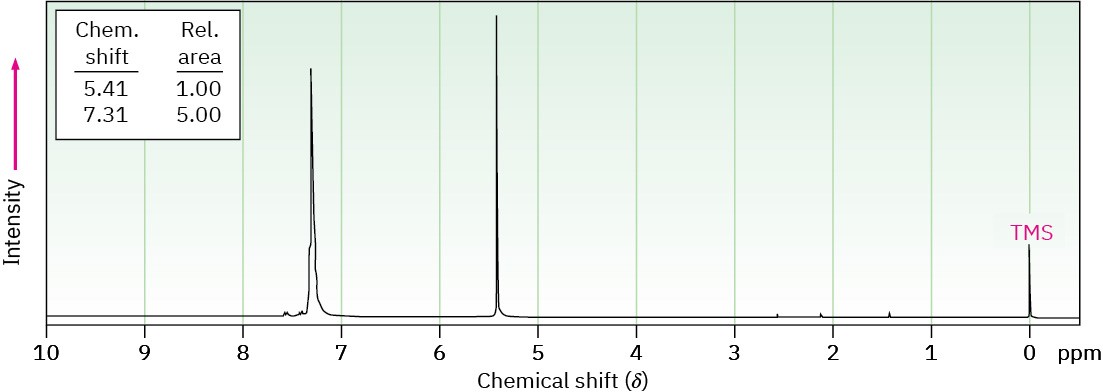
Problem 15-49
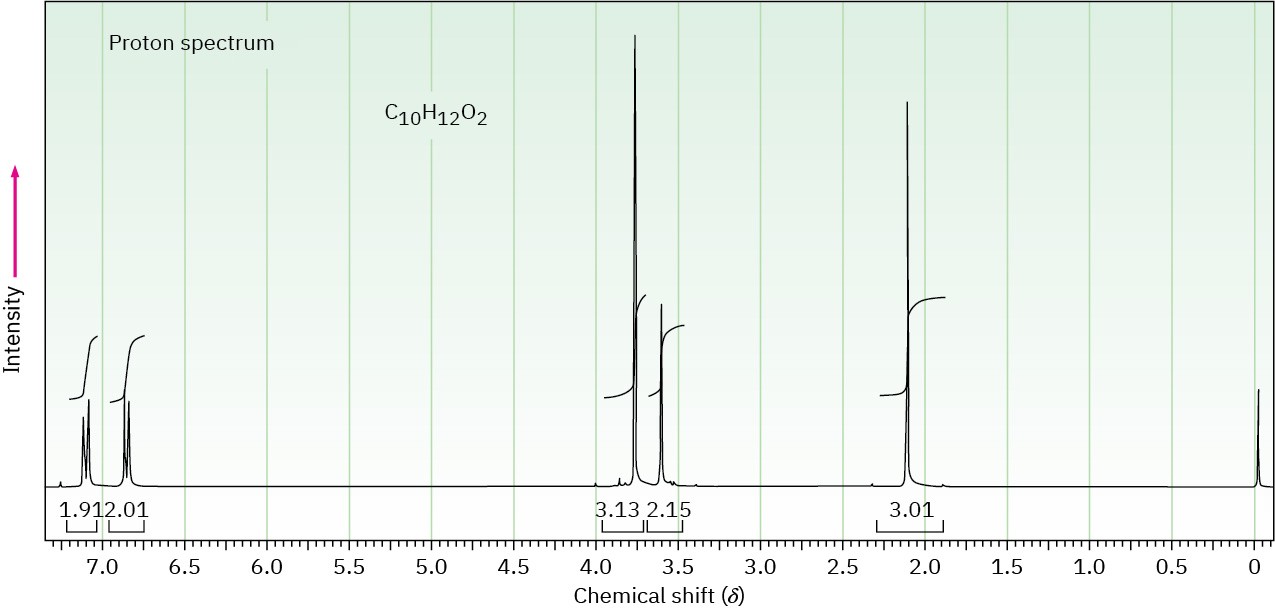
The proton NMR spectrum for a compound with formula C10H12O2 is shown. The infrared spectrum has a strong band at 1711 cm–1. The normal carbon-13 NMR spectral results are tabulated along with the DEPT-135 and DEPT-90 information. Draw the structure of this compound.
|
Normal Carbon |
DEPT-135 |
DEPT-90 |
|
29 ppm |
Positive |
No peak |
|
50 |
Negative |
No peak |
|
55 |
Positive |
No peak |
|
114 |
Positive |
Positive |
|
126 |
No peak |
No peak |
|
130 |
Positive |
Positive |
|
159 |
No peak |
No peak |
|
207 |
No peak |
No peak |
Problem 15-50
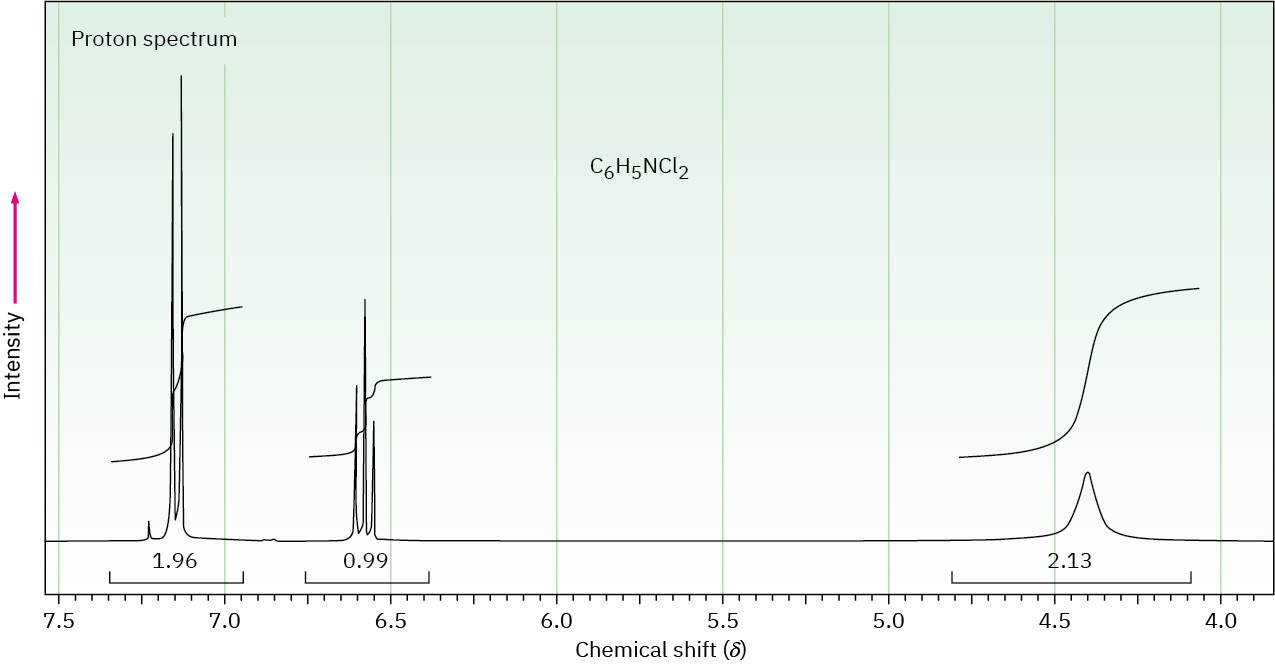
The proton NMR spectrum of a compound with formula C6H5NCl2 is shown. The normal carbon-13 and DEPT experimental results are tabulated. The infrared spectrum shows peaks at 3432 and 3313 cm–1 and a series of medium-sized peaks between 1618 and 1466 cm–1. Draw the structure of this compound.
|
Normal Carbon |
DEPT-135 |
DEPT-90 |
|
118.0 ppm |
Positive |
Positive |
|
119.5 |
No peak |
No peak |
|
128.0 |
Positive |
Positive |
|
140.0 |
No peak |
No peak |
Problem 15-51
Aromatic substitution reactions occur by addition of an electrophile such as Br+ to an aromatic ring to yield an allylic carbocation intermediate, followed by loss of H+. Show the structure of the intermediate formed by reaction of benzene with Br+.
Problem 15-52
The substitution reaction of toluene with Br2 can, in principle, lead to the formation of three isomeric bromotoluene products. In practice, however, only o– and p-bromotoluene are formed in substantial amounts. The meta isomer is not formed. Draw the structures of the three possible carbocation intermediates (Problem 51), and explain why ortho and para products predominate over meta products.
Problem 15-53
Look at the following aromatic anions and their linear counterparts, and draw all of the resonance forms for each. What patterns emerge?
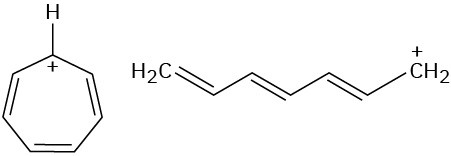
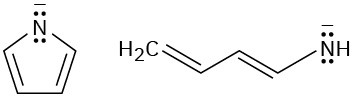
Problem 15-54
After the following reaction, the chemical shift of Ha moves downfield from 6.98 ppm to 7.30 ppm. Explain.
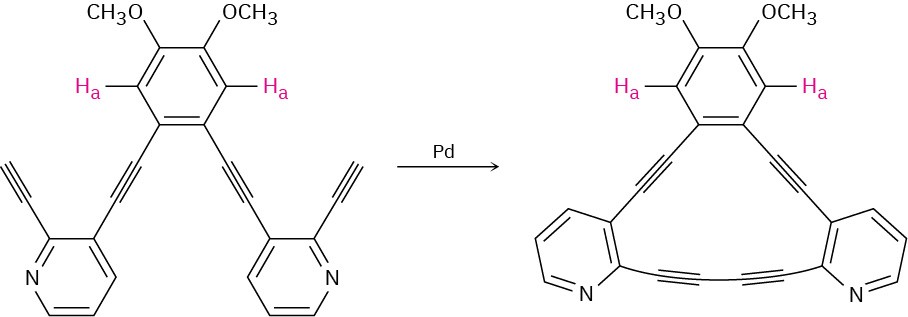
Problem 15-55
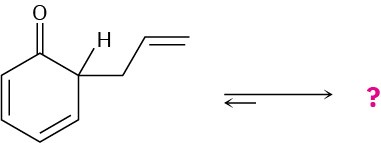
The following compound is the product initially formed in a Claisen rearrangement, which we’ll see in Section 18.4. This product is not isolated, but tautomerizes to its enol form. Give the structure of the enol and provide an explanation as to why the enol tautomer is favored.
Problem 15-56
Compounds called azo dyes are the major source of artificial color in textiles and food. Part of the reason for their intense coloring is the conjugation from an electron-donating group through the diazo bridge (−N=N−) to an electron-withdrawing group on the other side. For the following azo dyes, draw a resonance form that shows how the electron-donating group is related to the electron-withdrawing group on the other side of the diazo bridge. Used curved arrows to show how the electrons are reorganized.
(a)
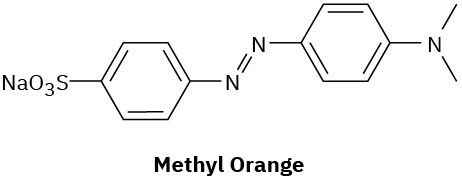
(b)