Infrared Spectroscopy
Carboxylic acids have two characteristic IR absorptions that make the –CO2H group easily identifiable. The O–H bond of the carboxyl group gives rise to a very broad absorption over the range 2500 to 3300 cm–1. The C═O bond shows an absorption between 1710 and 1760 cm–1. The exact position of C═O absorption depends both on the structure of the molecule and on whether the acid is free (monomeric) or hydrogen-bonded (dimeric). Free carboxyl groups absorb at 1760 cm–1, but the more commonly encountered dimeric carboxyl groups absorb in a broad band centered around 1710 cm–1. As with other carbonyl-containing functional groups, conjugation with an alkene or benzene ring lowers the frequency of the C═O stretch by 20 to 30 cm–1.

Both the broad O–H absorption and the C═O absorption at 1710 cm–1 (dimeric) are identified in the IR spectrum of butanoic acid shown in Figure 20.6.
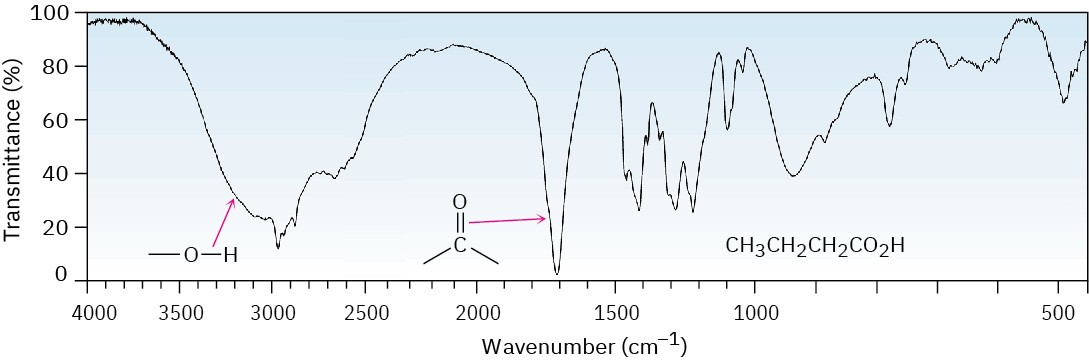
Figure 20.6 IR spectrum of butanoic acid.
Nitriles show an intense and easily recognizable C≡N bond absorption near 2250 cm–1 for saturated compounds and 2230 cm–1 for aromatic and conjugated molecules. Few other functional groups absorb in this region, so IR spectroscopy is highly diagnostic for nitriles.
Problem 20-15
Cyclopentanecarboxylic acid and 4-hydroxycyclohexanone have the same formula (C6H10O2), and both contain an –OH and a C═O group. How could you distinguish between them using IR spectroscopy?
Nuclear Magnetic Resonance Spectroscopy
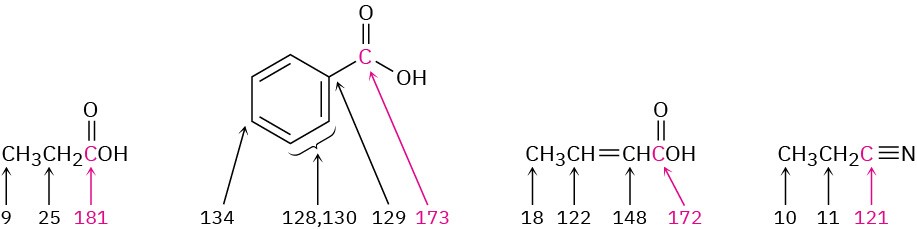
Carboxyl carbon atoms absorb in the range 165 to 185 δ in the 13C NMR spectrum, with aromatic and α,β-unsaturated acids near the upfield end of the range (∼165 δ) and saturated aliphatic acids near the downfield end (∼185 δ). Nitrile carbons absorb in the range 115 to 130 δ.
In the 1H NMR spectrum, the acidic –CO2H proton normally absorbs as a singlet near 12 δ. The chemical shift of the carboxyl proton is concentration and solvent dependent, as these variables can change the extent of hydrogen-bonding in the sample. In some cases, the carboxyl-proton resonance is broadened to the point of being nearly undetectable. Traces of water in the sample can exacerbate the situation. As with alcohols (Section 17.11), the – CO2H proton can be replaced by deuterium when D2O is added to the sample tube, causing the absorption to disappear from the NMR spectrum. Figure 20.7 shows the 1H NMR spectrum of phenylacetic acid. Note that the –CO2H absorption occurs at 12.0 δ.
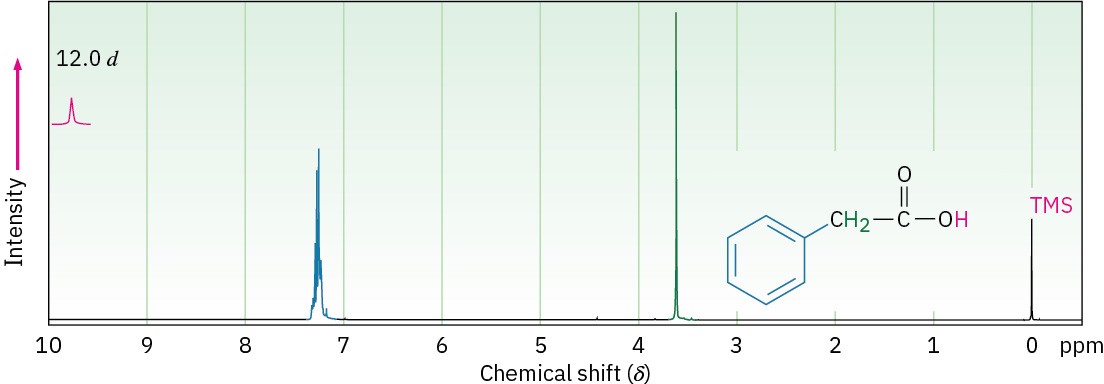
Figure 20.7 Proton NMR spectrum of phenylacetic acid, C6H5CH2CO2H.
Problem 20-16
How could you distinguish between the isomers cyclopentanecarboxylic acid and 4- hydroxycyclohexanone by 1H and 13C NMR spectroscopy? (See Problem 20-15.)

