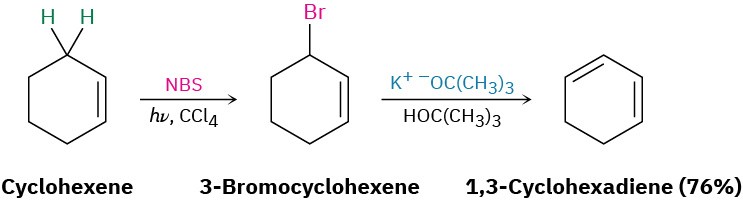
Conjugated dienes can be prepared by some of the methods previously discussed for preparing alkenes (Section 11.7–Section 11.11). The base-induced elimination of HX from an allylic halide is one such reaction.
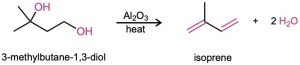
Simple conjugated dienes used in polymer synthesis include 1,3-butadiene, chloroprene (2-chloro-1,3-butadiene), and isoprene (2-methyl-1,3-butadiene). Isoprene has been prepared industrially by several methods, including the acid-catalyzed double dehydration of 3-methyl-1,3-butanediol.
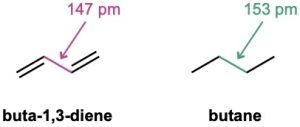
One of the properties that distinguishes conjugated from nonconjugated dienes is that the central single bond is shorter than might be expected. The C2–C3 single bond in 1,3- butadiene, for instance, has a length of 147 pm, some 6 pm shorter than the C2–C3 bond in butane (153 pm).
Another distinctive property of conjugated dienes is their unusual stability, as evidenced by their heats of hydrogenation (Table 14.1). We saw in Section 7.6 that monosubstituted alkenes, such as 1-butene, have Δ𝐻∘hydrog near –126 kJ/mol (–30.1 kcal/mol), whereas disubstituted alkenes, such as 2-methylpropene, have Δ𝐻∘hydrog near –119 kJ/mol (–28.4 kcal/mol), which is approximately 7 kJ/mol less negative. We concluded from these data that more highly substituted alkenes are more stable than less substituted ones. That is, more highly substituted alkenes release less heat on hydrogenation because they contain less energy to start with. A similar conclusion can be drawn for conjugated dienes.
Table 14.1 Heats of Hydrogenation for Some Alkenes and Dienes
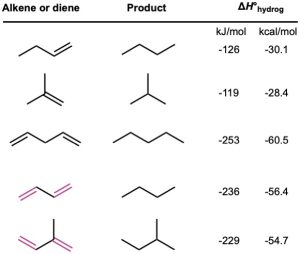
Because a monosubstituted alkene has a Δ𝐻∘hydrog of approximately –126 kJ/mol, we might expect that a compound with two monosubstituted double bonds would have a Δ𝐻∘hydrog approximately twice that value, or –252 kJ/mol. Nonconjugated dienes, such as 1,4- pentadiene (Δ𝐻∘hydrog = −253 kJ/mol), meet this expectation, but the conjugated diene 1,3-butadiene (Δ𝐻∘hydrog = −236 kJ/mol) does not. 1,3-Butadiene is approximately 16 kJ/mol (3.8 kcal/mol) more stable than expected.

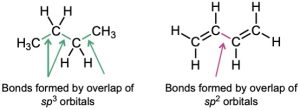
What accounts for the stability of conjugated dienes? According to valence bond theory (Section 1.5 and Section 1.8), their stability is due to orbital hybridization. Typical C–C single bonds, like those in alkanes, result from σ overlap of sp3 orbitals on both carbons, but in a conjugated diene, the central C–C single bond results from σ overlap of sp2 orbitals on both carbons. Because sp2 orbitals have more s character (33% s) than sp3 orbitals (25% s), the electrons in sp2 orbitals are closer to the nucleus and the bonds they form are somewhat shorter and stronger. Thus, the “extra” stability of a conjugated diene results in part from the greater amount of s character in the orbitals forming the C–C single bond.
According to molecular orbital theory (Section 1.11), the stability of conjugated dienes arises because of an interaction between the π orbitals of the two double bonds. To review briefly, when two p atomic orbitals combine to form a π bond, two π molecular orbitals (MOs) result. One is lower in energy than the starting p orbitals and is therefore bonding; the other is higher in energy, has a node between nuclei, and is antibonding. The two π electrons occupy the low-energy, bonding orbital, resulting in formation of a stable bond between atoms (Figure 14.2).
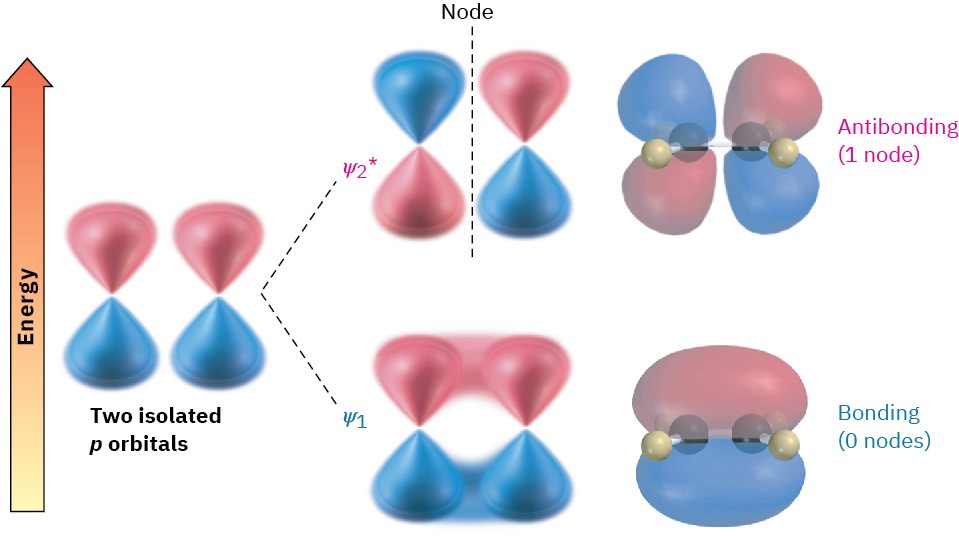
Figure 14.2 Two p orbitals combine to form two π molecular orbitals. Both electrons occupy the low-energy, bonding orbital, leading to a net lowering of energy and formation of a stable bond. The asterisk on 𝜓2∗ indicates an antibonding orbital.
Now let’s combine four adjacent p atomic orbitals, as occurs in a conjugated diene. In so doing, we generate a set of four π molecular orbitals, two of which are bonding and two of which are antibonding (Figure 14.3). The four π electrons occupy the two bonding orbitals, leaving the antibonding orbitals vacant.
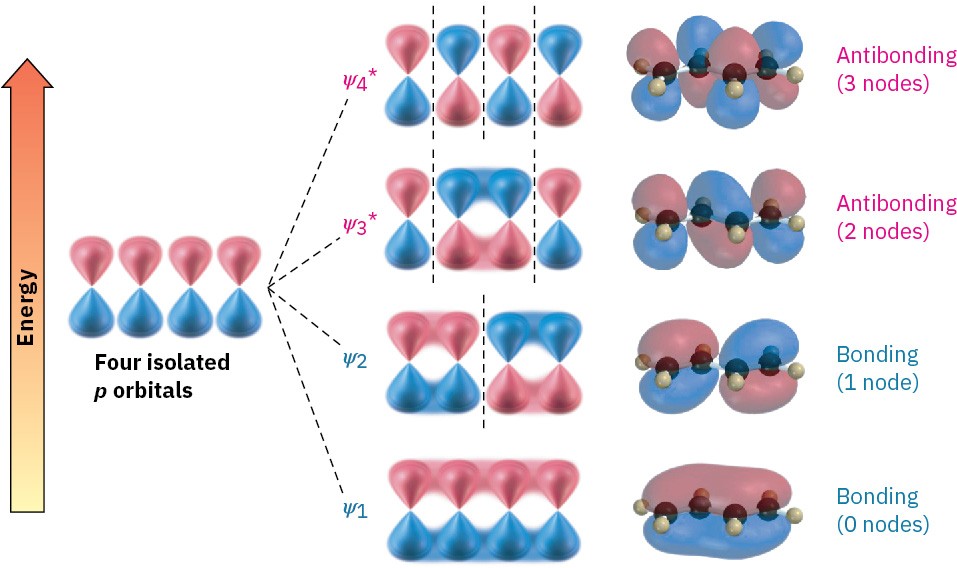
Figure 14.3 Four π molecular orbitals in 1,3-butadiene. Note that the number of nodes between nuclei increases as the energy level of the orbital increases.
The lowest-energy π molecular orbital (denoted ψ1, Greek psi) has no nodes between the nuclei and is therefore bonding. The π MO of next-lowest energy, ψ2, has one node between nuclei and is also bonding. Above ψ1 and ψ2 in energy are the two antibonding π MOs, 𝜓3∗ and 𝜓4∗. (The asterisks indicate antibonding orbitals.) Note that the number of nodes between nuclei increases as the energy level of the orbital increases. The 𝜓3∗ orbital has two nodes between nuclei, and 𝜓4∗, the highest-energy MO, has three nodes between nuclei.
Comparing the π molecular orbitals of 1,3-butadiene (two conjugated double bonds) with those of 1,4-pentadiene (two isolated double bonds) shows why the conjugated diene is more stable. In a conjugated diene, the lowest-energy π MO (ψ1) has a favorable bonding interaction between C2 and C3 that is absent in a nonconjugated diene. As a result, there is a certain amount of double-bond character to the C2–C3 single bond, making that bond both stronger and shorter than a typical single bond. Electrostatic potential maps show clearly the additional electron density in the central single bond (Figure 14.4).
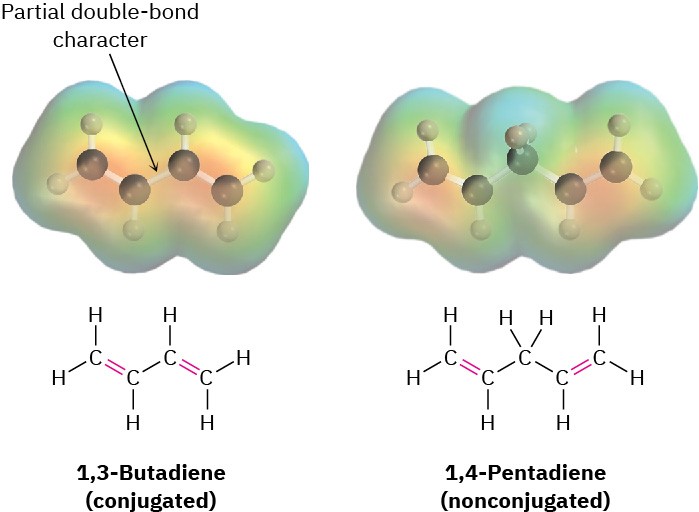
Figure 14.4 Electrostatic potential maps of 1,3-butadiene (conjugated) and 1,4- pentadiene (nonconjugated). Additional electron density is present in the central C−C bond of 1,3-butadiene, corresponding to partial double-bond character.
In describing 1,3-butadiene, we say that the π electrons are spread out, or delocalized, over the entire π framework, rather than localized between two specific nuclei. Delocalization allows the bonding electrons to be closer to more nuclei, thus leading to lower energy and greater stability.
Problem 14-1
Allene, H2C=C=CH2, has a heat of hydrogenation of –298 kJ/mol (–71.3 kcal/mol). Rank a conjugated diene, a nonconjugated diene, and an allene in order of stability.

