How can we predict whether conrotatory or disrotatory motion will occur in a given case? According to frontier orbital theory, the stereochemistry of an electrocyclic reaction is determined by the symmetry of the polyene HOMO. The electrons in the HOMO are the highest-energy, most loosely held electrons and are therefore most easily moved during reaction. For thermal reactions, the ground-state electron configuration is used to identify the HOMO; for photochemical reactions, the excited-state electron configuration is used.
Let’s look again at the thermal ring-closure of conjugated trienes. According to Figure 30.3, the HOMO of a conjugated triene in its ground state has lobes of like sign on the same side of the molecule, a symmetry that predicts disrotatory ring-closure. This disrotatory cyclization is precisely what is observed in the thermal cyclization of 2,4,6-octatriene. The 2E,4Z,6E isomer yields cis product; the 2E,4Z,6Z isomer yields trans product (Figure 30.6).
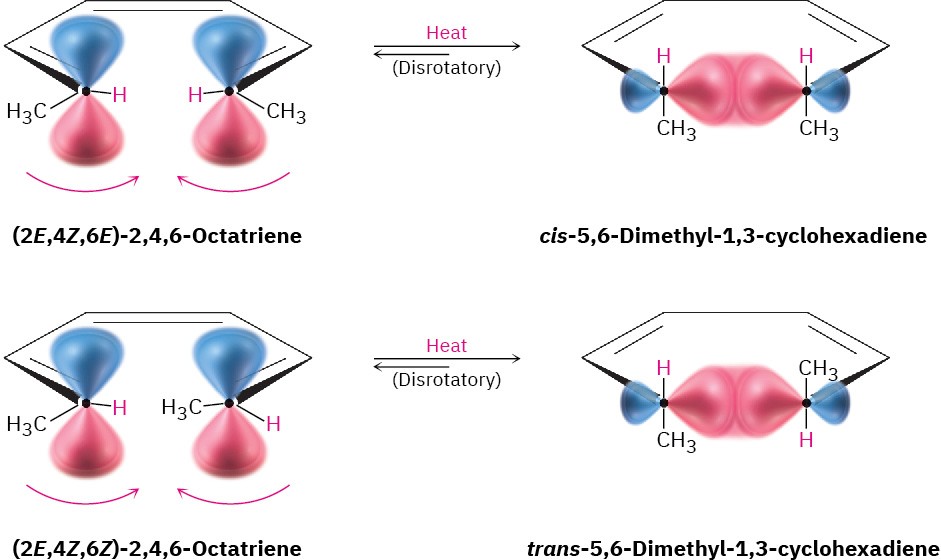
Figure 30.6 Thermal cyclizations of 2,4,6-octatrienes occur by disrotatory ring-closures.
In the same way, the ground-state HOMO of conjugated dienes (Figure 30.2) has a symmetry that predicts conrotatory ring-closure. In practice, however, the conjugated diene reaction can be observed only in the reverse direction (cyclobutene → diene) because of the position of the equilibrium. We therefore find that the 3,4-dimethylcyclobutene ring opens in a conrotatory fashion. cis-3,4-Dimethylcyclobutene yields (2E,4Z)-2,4-hexadiene, and trans-3,4-dimethylcyclobutene yields (2E,4E)-2,4-hexadiene by conrotatory opening (Figure 30.7).
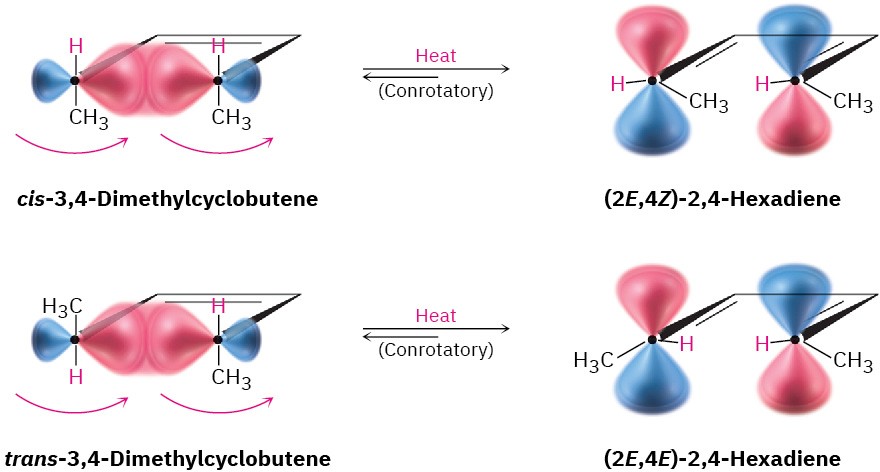
Figure 30.7 Thermal ring-openings of cis– and trans-dimethylcyclobutene occur by conrotatory paths.
Note that a conjugated diene and a conjugated triene react with opposite stereochemistry. The diene opens and closes by a conrotatory path, whereas the triene opens and closes by a disrotatory path. This is due to the different symmetries of the diene and triene HOMOs.
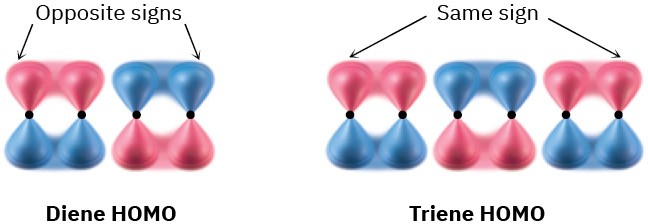
It turns out that there is an alternating relationship between the number of electron pairs (double bonds) undergoing bond reorganization and the stereochemistry of ring-opening or -closure. Polyenes with an even number of electron pairs undergo thermal electrocyclic reactions in a conrotatory sense, whereas polyenes with an odd number of electron pairs undergo the same reactions in a disrotatory sense.
Problem 30-2
Draw the products you would expect from conrotatory and disrotatory cyclizations of (2Z,4Z,6Z)-2,4,6-octatriene. Which of the two paths would you expect the thermal reaction to follow?
Problem 30-3
trans-3,4-Dimethylcyclobutene can open by two conrotatory paths to give either (2E,4E)-2,4-hexadiene or (2Z,4Z)-2,4-hexadiene. Explain why both products are symmetry-allowed, and then account for the fact that only the 2E,4E isomer is obtained in practice.

