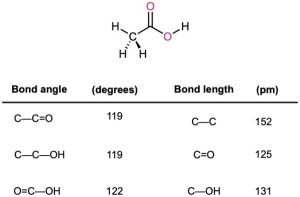
Carboxylic acids are similar in some respects to both ketones and alcohols. Like ketones, the carboxyl carbon is sp2-hybridized, and carboxylic acid groups are therefore planar with C–C═O and O═C–O bond angles of approximately 120° (Table 20.2).
Table 20.2 Physical Parameters for Acetic Acid
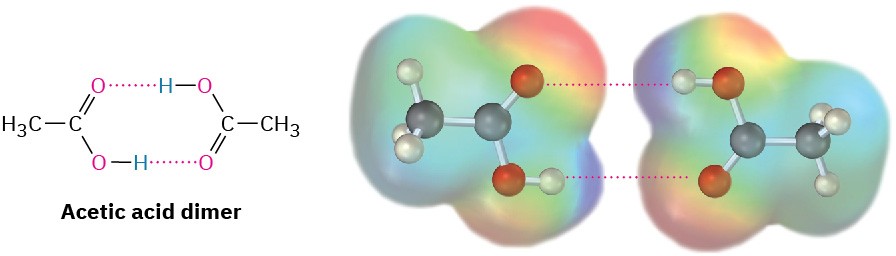
Like alcohols, carboxylic acids are strongly associated because of hydrogen-bonding. Most carboxylic acids exist as cyclic dimers held together by two hydrogen bonds. This strong hydrogen-bonding has a noticeable effect on boiling points, making carboxylic acids boil far less easily than their corresponding alcohols. Acetic acid, for instance, has a boiling point of 118 °C, versus 78 °C for ethanol, even though both compounds have two carbons.
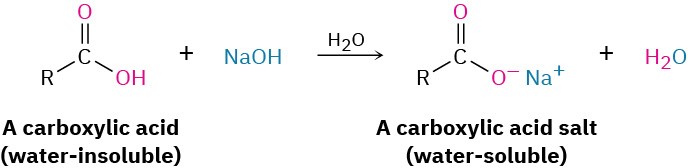
The most obvious property of carboxylic acids is implied by their name: carboxylic acids are acidic. They therefore react with bases such as NaOH and NaHCO3 to give metal carboxylate salts, RCO2− M+. Carboxylic acids with more than six carbons are only slightly soluble in water, but the alkali metal salts of carboxylic acids are often highly water-soluble. In fact, it’s often possible to purify an acid by extracting its salt into aqueous base, then reacidifying and extracting the pure acid back into an organic solvent.
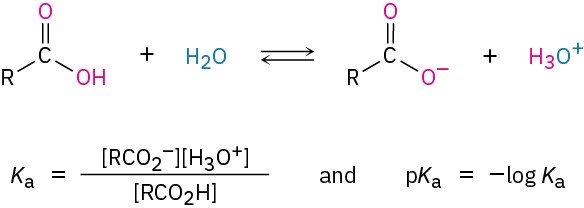
Like other Brønsted–Lowry acids discussed in Section 2.7, carboxylic acids dissociate slightly in dilute aqueous solution to give H3O+ and the corresponding carboxylate anions, RCO2–. The extent of dissociation is given by an acidity constant, Ka.
A list of Ka values for various carboxylic acids is given in Table 20.3. For most, Ka is approximately 10–4 to 10–5. Acetic acid, for instance, has Ka = 1.75 × 10–5 at 25 °C, which corresponds to a pKa of 4.76. In practical terms, a Ka value near 10–5 means that only about 0.1% of the molecules in a 0.1 M solution are dissociated, as opposed to the 100% dissociation found with strong mineral acids like HCl.
Table 20.3 Acidity of Some Carboxylic Acids
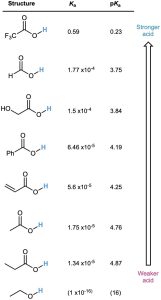
Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols. The Ka of ethanol, for example, is approximately 10–16, making it a weaker acid than acetic acid by a factor of 1011.
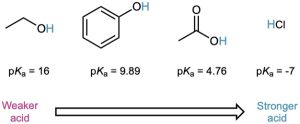
Why are carboxylic acids so much more acidic than alcohols, even though both contain –OH groups? An alcohol dissociates to give an alkoxide ion, in which the negative charge is localized on a single electronegative atom. A carboxylic acid, however, gives a carboxylate ion, in which the negative charge is delocalized over two equivalent oxygen atoms (Figure 20.2). In resonance terms (Section 2.4), a carboxylate ion is a stabilized resonance hybrid of two equivalent structures. Since a carboxylate ion is more stable than an alkoxide ion, it is lower in energy and more favored in the dissociation equilibrium.
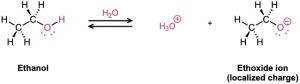
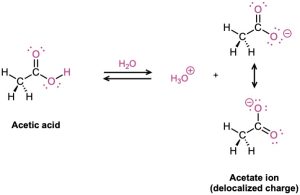
Figure 20.2 An alkoxide ion has its charge localized on one oxygen atom and is less stable, while a carboxylate ion has the charge spread equally over both oxygens and is therefore more stable.
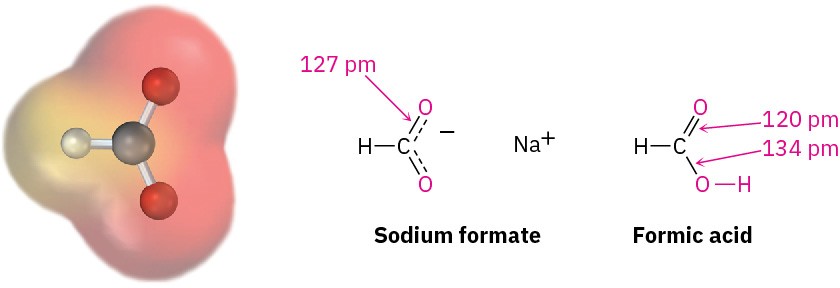
Experimental evidence for the equivalence of the two carboxylate oxygens comes from X-ray crystallographic studies on sodium formate. Both carbon–oxygen bonds are 127 pm in length, midway between the C═O double bond (120 pm) and the C–O single bond (134 pm) of formic acid. An electrostatic potential map of the formate ion also shows how the negative charge (red) is spread equally over both oxygens.
Problem 20-3
Assume you have a mixture of naphthalene and benzoic acid that you want to separate. How might you take advantage of the acidity of one component in the mixture to effect a separation?
Problem 20-4
The Ka for dichloroacetic acid is 3.32 × 10–2. Approximately what percentage of the acid is dissociated in a 0.10 M aqueous solution?

