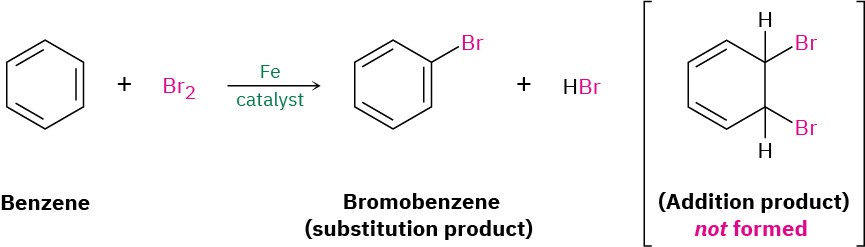
Benzene (C6H6) has six fewer hydrogens than the six-carbon cycloalkane cyclohexane (C6H12) and is clearly unsaturated, usually being represented as a six-membered ring with alternating double and single bonds. Yet it has been known since the mid-1800s that benzene is much less reactive than typical alkenes and fails to undergo typical alkene addition reactions. Cyclohexene, for instance, reacts rapidly with Br2 and gives the addition product 1,2-dibromocyclohexane, but benzene reacts slowly with Br2 and gives the substitution product C6H5Br rather than the addition product.
We can get a quantitative idea of benzene’s stability by measuring heats of hydrogenation (Section 7.6). Cyclohexene, an isolated alkene, has Δ𝐻∘hydrog =– 118 kJ/mol (–28.2 kcal/mol), and 1,3-cyclohexadiene, a conjugated diene, has Δ𝐻∘hydrog =– 230 kJ/mol (–55.0 kcal/mol). As noted in Section 14.1, this value for 1,3-cyclohexadiene is a bit less than twice that for cyclohexene because conjugated dienes are more stable than isolated dienes.
Carrying the process one step further, we might expect Δ𝐻∘hydrog for “cyclohexatriene” (benzene) to be a bit less than 3 × 118 = 354 kJ/mol, or three times the cyclohexene value. The actual value, however, is –206 kJ/mol, some 148 kJ/mol (35.4 kcal/mol) less than expected. Because of this difference in actual and expected energy released during hydrogenation, benzene must have 148 kJ/mol less energy to begin with. In other words, benzene is more stable than expected by 148 kJ/mol (Figure 15.3).
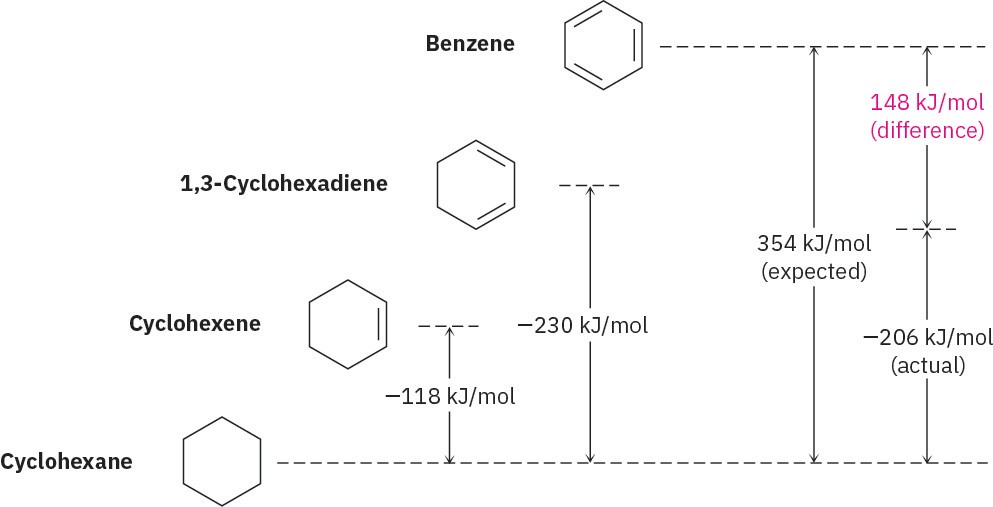
Figure 15.3 A comparison of the heats of hydrogenation for cyclohexene, 1,3- cyclohexadiene, and benzene. Benzene is 148 kJ/mol (35.4 kcal/mol) more stable than might be expected for “cyclohexatriene.”
Further evidence for the unusual nature of benzene is that all its carbon–carbon bonds have the same length—139 pm, which is intermediate between typical single (154 pm) and double (134 pm) bonds. In addition, an electrostatic potential map shows that the electron density in all six C–C bonds is identical. Thus, benzene is a planar molecule with the shape of a regular hexagon. All C–C–C bond angles are 120°, all six carbon atoms are sp2– hybridized, and each carbon has a p orbital perpendicular to the plane of the six-membered ring.
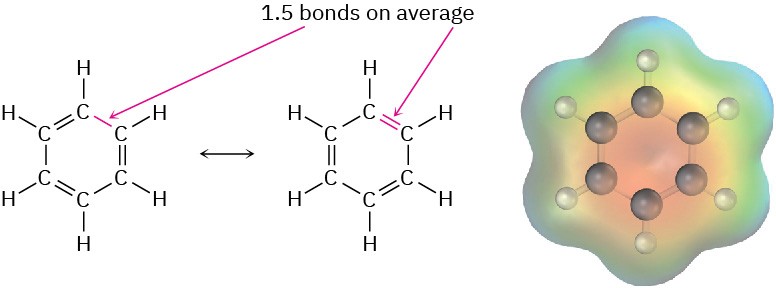
Because all six carbon atoms and all six p orbitals in benzene are equivalent, it’s impossible to define three localized π bonds in which a given p orbital overlaps only one neighboring p orbital. Rather, each p orbital overlaps equally well with both neighboring p orbitals, leading to a picture of benzene in which all six π electrons are free to move about the entire ring (Figure 15.4b). In resonance terms (Section 2.4 and Section 2.5), benzene is a hybrid of two equivalent forms. Neither form is correct by itself; the true structure of benzene is somewhere in between the two resonance forms but is impossible to draw with our usual conventions. Because of this resonance, benzene is more stable and less reactive than a typical alkene.
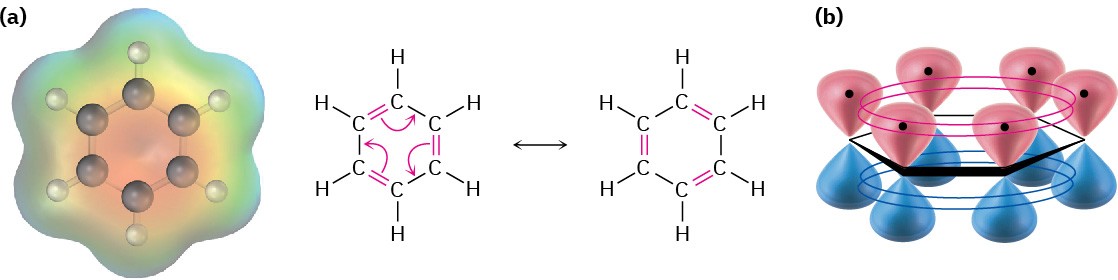
Figure 15.4 (a) An electrostatic potential map of benzene and (b) an orbital picture. Each of the six carbon atoms has a p orbital that can overlap equally well with neighboring p orbitals on both sides. As a result, all C–C bonds are equivalent and benzene must be represented as a hybrid of two resonance forms.
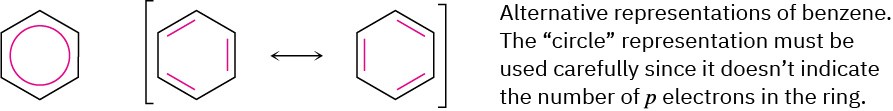
Chemists sometimes represent the two benzene resonance forms by using a circle to indicate the equivalence of the carbon–carbon bonds, but this representation has to be
used carefully because it doesn’t indicate the number of π electrons in the ring. (How many electrons does a circle represent?) In this book, benzene and other aromatic compounds will be represented by single line-bond structures. We’ll be able to keep count of π electrons this way but must be aware of the limitations of the drawings.
Having just seen a resonance description of benzene, let’s now look at the alternative molecular orbital description. We can construct π molecular orbitals for benzene just as we did for 1,3-butadiene in Section 14.1. If six p atomic orbitals combine in a cyclic manner, six benzene molecular orbitals result, as shown in Figure 15.5. The three low-energy molecular orbitals, denoted ψ1, ψ2, and ψ3, are bonding combinations, and the three high-energy orbitals are antibonding.
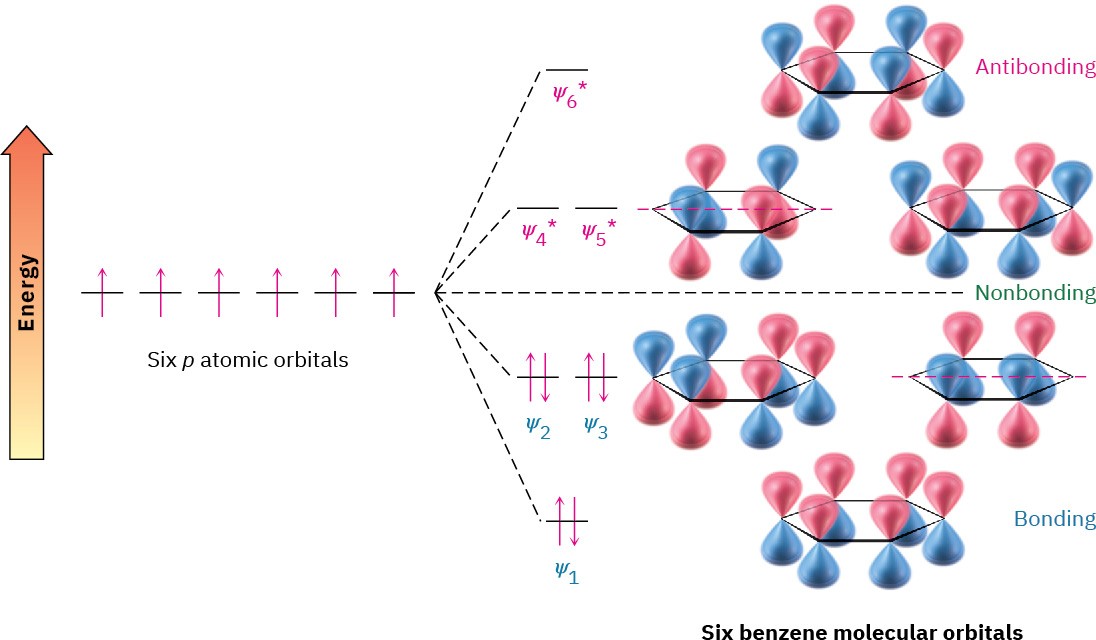
Figure 15.5 The six benzene π molecular orbitals. The bonding orbitals ψ2 and ψ3 have the same energy and are said to be degenerate, as are the antibonding orbitals ψ4* and ψ5*. The orbitals ψ3 and ψ4* have no π electron density on two carbons because of a node passing through these atoms.
Note that the two bonding orbitals ψ2 and ψ3 have the same energy, as do the two antibonding orbitals 𝜓4* and 𝜓5∗. Such orbitals with the same energy are said to be degenerate. Note also that the two orbitals ψ3 and 𝜓4∗ have nodes passing through ring carbon atoms, thereby leaving no π electron density on these carbons. The six p electrons of benzene occupy the three bonding molecular orbitals and are delocalized over the entire conjugated system, leading to the observed 150 kJ/mol stabilization of benzene.
Problem 15-4
Pyridine is a flat, hexagonal molecule with bond angles of 120°. It undergoes substitution rather than addition and generally behaves like benzene. Draw a picture of the π orbitals of pyridine to explain its properties. Check your answer by looking ahead to Section 15.5.