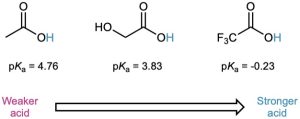
The listing of pKa values shown previously in Table 20.3 indicates that there are substantial differences in acidity from one carboxylic acid to another. For example, trifluoroacetic acid (Ka = 0.59) is 33,000 times as strong as acetic acid (Ka = 1.75 × 10–5). How can we account for such differences?
Because the dissociation of a carboxylic acid is an equilibrium process, any factor that stabilizes the carboxylate anion relative to undissociated carboxylic acid will drive the equilibrium toward increased dissociation and result in increased acidity. For instance, three electron-withdrawing fluorine atoms delocalize the negative charge in the trifluoroacetate anion, thereby stabilizing the ion and increasing the acidity of CF3CO2H. In the same way, glycolic acid (HOCH2CO2H; pKa = 3.83) is stronger than acetic acid because of the electron-withdrawing effect of the electronegative oxygen atom.
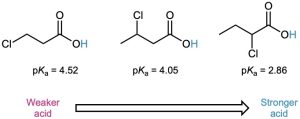
Because inductive effects operate through σ bonds and are dependent on distance, the effect of halogen substitution decreases as the substituent moves farther from the carboxyl. Thus, 2-chlorobutanoic acid has pKa = 2.86, 3-chlorobutanoic acid has pKa = 4.05, and 4-chlorobutanoic acid has pKa = 4.52, similar to that of butanoic acid itself.
Substituent effects on acidity are also found in substituted benzoic acids. We said during the discussion of electrophilic aromatic substitution in Section 16.4 that substituents on the aromatic ring strongly affect reactivity. Aromatic rings with electron-donating groups are activated toward further electrophilic substitution, and aromatic rings with electron- withdrawing groups are deactivated. Exactly the same effects can be observed on the acidity of substituted benzoic acids (Table 20.4).
Table 20.4 Substituent Effects on the Acidity of p-Substituted Benzoic Acids
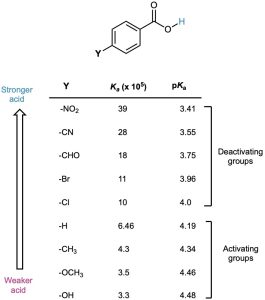
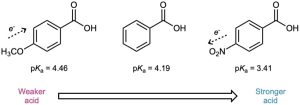
As Table 20.4 shows, an electron-donating (activating) group such as methoxy decreases acidity by destabilizing the carboxylate anion, and an electron-withdrawing (deactivating) group such as nitro increases acidity by stabilizing the carboxylate anion.
Because it’s much easier to measure the acidity of a substituted benzoic acid than it is to determine the relative reactivity of an aromatic ring toward electrophilic substitution, the correlation between the two effects is useful for predicting reactivity. If we want to know the effect of a certain substituent on electrophilic reactivity, we can simply find the acidity of the corresponding benzoic acid. Worked Example 20.1 gives an illustration.

Worked Example 20.1 Predicting the Effect of a Substituent on the Reactivity of an Aromatic Ring toward Electrophilic Substitution
The pKa of p-(trifluoromethyl)benzoic acid is 3.6. Is the trifluoromethyl substituent an activating or deactivating group in electrophilic aromatic substitution?
Strategy
Decide whether p-(trifluoromethyl)benzoic acid is stronger or weaker than benzoic acid. A substituent that strengthens the acid is a deactivating group because it withdraws electrons, and a substituent that weakens the acid is an activating group because it donates electrons.
Solution
A pKa of 3.6 means that p-(trifluoromethyl)benzoic acid is stronger than benzoic acid, whose pKa is 4.19. Thus, the trifluoromethyl substituent favors dissociation by helping stabilize the negative charge. Trifluoromethyl must therefore be an electron-withdrawing, deactivating group.
Problem 20-6
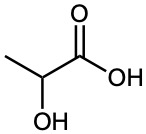
Which would you expect to be a stronger acid, the lactic acid found in tired muscles or acetic acid? Explain.
Problem 20-7
Dicarboxylic acids have two dissociation constants, one for the initial dissociation into a monoanion and one for the second dissociation into a dianion. For oxalic acid, HO2C–CO2H, the first ionization constant is pKa1 = 1.2 and the second ionization constant is pKa2 = 4.2.
Why is the second carboxyl group far less acidic than the first?
Problem 20-8
The pKa of p-cyclopropylbenzoic acid is 4.45. Is cyclopropylbenzene likely to be more reactive or less reactive than benzene toward electrophilic bromination? Explain.
Problem 20-9
Rank the following compounds in order of increasing acidity. Don’t look at a table of pKa data to help with your answer.
(a) benzoic acid, p-methylbenzoic acid, p-chlorobenzoic acid
(b) p-nitrobenzoic acid, acetic acid, benzoic acid

