The purpose of this chapter has been to get you up to speed—to review some ideas about atoms, bonds, and molecular geometry. As we’ve seen, organic chemistry is the study of carbon compounds. Although a division into organic and inorganic chemistry occurred historically, there is no scientific reason for the division.
An atom consists of a positively charged nucleus surrounded by one or more negatively charged electrons. The electronic structure of an atom can be described by a quantum mechanical wave equation, in which electrons are considered to occupy orbitals around the nucleus. Different orbitals have different energy levels and different shapes. For example, s orbitals are spherical and p orbitals are dumbbell-shaped. The ground-state electron configuration of an atom can be found by assigning electrons to the proper orbitals, beginning with the lowest-energy ones.
A covalent bond is formed when an electron pair is shared between atoms. According to valence bond (VB) theory, electron sharing occurs by the overlap of two atomic orbitals. According to molecular orbital (MO) theory, bonds result from the mathematical combination of atomic orbitals to give molecular orbitals, which belong to the entire molecule. Bonds that have a circular cross-section and are formed by head-on interaction are called sigma (σ) bonds; bonds formed by sideways interaction of p orbitals are called pi (π) bonds.
In the valence bond description, carbon uses hybrid orbitals to form bonds in organic molecules. When forming only single bonds with tetrahedral geometry, carbon uses four equivalent sp3 hybrid orbitals. When forming a double bond with planar geometry, carbon uses three equivalent sp2 hybrid orbitals and one unhybridized p orbital. When forming a triple bond with linear geometry, carbon uses two equivalent sp hybrid orbitals and two unhybridized p orbitals. Other atoms such as nitrogen, phosphorus, oxygen, and sulfur also use hybrid orbitals to form strong, oriented bonds.
Organic molecules are usually drawn using either condensed structures or skeletal structures. In condensed structures, carbon–carbon and carbon–hydrogen bonds aren’t shown. In line structures, only the bonds and not the atoms are shown. A carbon atom is assumed to be at the ends and at the junctions of lines (bonds), and the correct number of hydrogens is supplied mentally.
Why You Should Work Problems – There’s no surer way to learn organic chemistry than by working problems. Although careful reading and rereading of this text are important, reading alone isn’t enough. You must also be able to use the information you’ve read and be able to apply your knowledge in new situations. Working problems gives you practice at doing this.
Each chapter in this book provides many problems of different sorts. The in-chapter problems are placed for immediate reinforcement of ideas just learned, while end-of- chapter problems provide additional practice and come in several forms. They often begin with a short section called “Visualizing Chemistry,” which helps you see the microscopic world of molecules and provides practice for working in three dimensions. After the visualizations are many further problems, which are organized by topic. Early problems are primarily of the drill type, providing an opportunity for you to practice your command of the fundamentals. Later problems tend to be more thought-provoking, and some are real challenges. As you study organic chemistry, take the time to work the problems. Do the ones you can, and ask for help on the ones you can’t. If you’re stumped by a particular problem, check the accompanying Study Guide and Student Solutions Manual for an explanation that should help clarify the difficulty. Working problems takes effort, but the payoff in knowledge and understanding is immense.
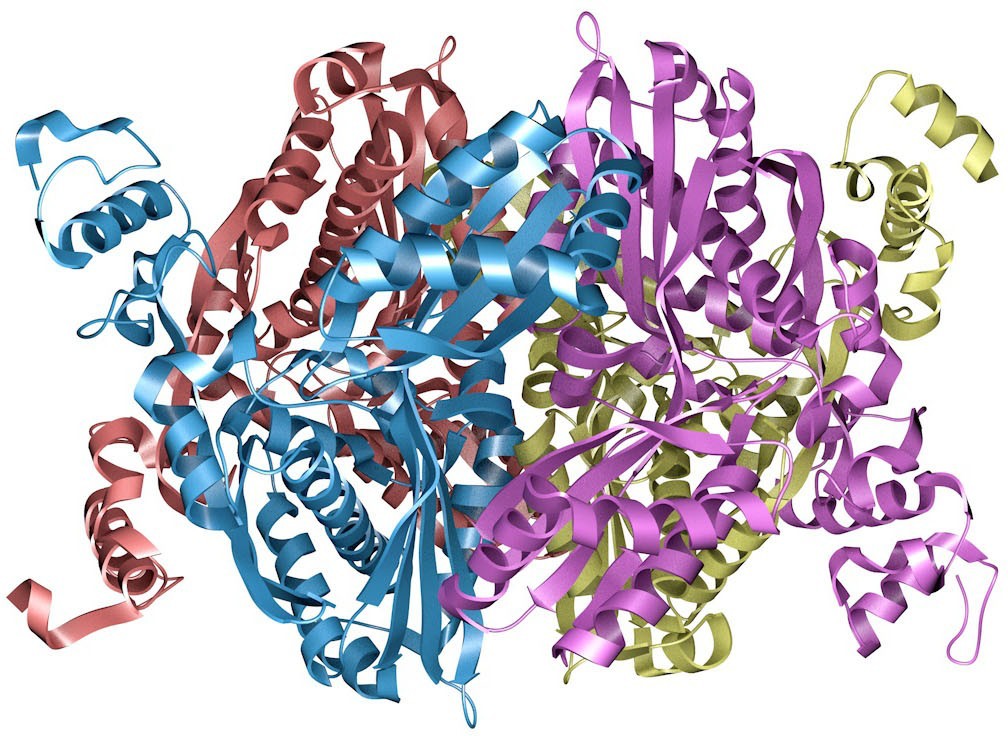
Figure 1.1 The enzyme HMG–CoA reductase, shown here as a so-called ribbon model, catalyzes a crucial step in the body’s synthesis of cholesterol. Understanding how this enzyme functions has led to the development of drugs credited with saving millions of lives. (credit: image from the RCSB PDB (rcsb.org) of PBD ID 1HW9 (E.S. Istvan,
J. Deisenhofer) (2001) Structural mechanism for statin inhibition of HMG-CoA reductase
Science 292: 1160–1164/RCSB PDB, CC BY 1.0)
Chapter Contents
- Atomic Structure: The Nucleus
- Atomic Structure: Orbitals
- Atomic Structure: Electron Configurations
- Development of Chemical Bonding Theory
- Describing Chemical Bonds: Valence Bond Theory
- sp3 Hybrid Orbitals and the Structure of Methane
- sp3 Hybrid Orbitals and the Structure of Ethane
- sp2 Hybrid Orbitals and the Structure of Ethylene
- sp Hybrid Orbitals and the Structure of Acetylene
- Hybridization of Nitrogen, Oxygen, Phosphorus, and Sulfur
- Describing Chemical Bonds: Molecular Orbital Theory
- Drawing Chemical Structures

