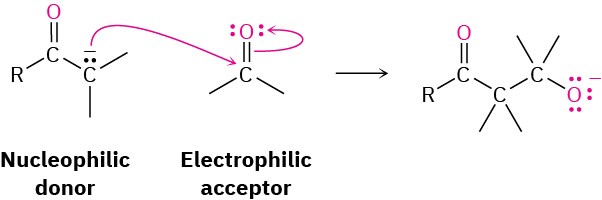
In this chapter, we’ve discussed the fourth and last of the common carbonyl-group reactions—the carbonyl condensation. A carbonyl condensation reaction takes place between two carbonyl partners and involves both nucleophilic addition and α-substitution processes. One carbonyl partner is converted by base into a nucleophilic enolate ion, which then adds to the electrophilic carbonyl group of the second partner. The first partner thus undergoes an α substitution, while the second undergoes a nucleophilic addition.
The aldol reaction is a carbonyl condensation that occurs between two aldehyde or ketone molecules. Aldol reactions are reversible, leading first to β-hydroxy aldehydes/ketones and then to α,β-unsaturated products after dehydration. Mixed aldol condensations between two different aldehydes or ketones generally give a mixture of all four possible products. A mixed reaction can be successful, however, if one of the two partners is an unusually good donor (ethyl acetoacetate, for instance) or if it can act only as an acceptor (formaldehyde and benzaldehyde, for instance). Intramolecular aldol condensations of 1,4- and 1,5-diketones are also successful and provide a good way to make five- and six-membered rings.
The Claisen condensation reaction is a carbonyl condensation that occurs between two ester components and gives a β-keto ester product. Mixed Claisen condensations between two different esters are successful only when one of the two partners has no acidic α hydrogens (ethyl benzoate and ethyl formate, for instance) and thus can function only as the acceptor partner. Intramolecular Claisen condensations, called Dieckmann cyclization reactions, yield five- and six-membered cyclic β-keto esters starting from 1,6- and 1,7-diesters.
The conjugate addition of a carbon nucleophile to an α,β-unsaturated acceptor is known as the Michael reaction. The best Michael reactions take place between relatively acidic donors (β-keto esters or β-diketones) and unhindered α,β-unsaturated acceptors.
Enamines, prepared by reaction of a ketone with a disubstituted amine, are also good Michael donors in the Stork enamine reaction.
Carbonyl condensation reactions are widely used in synthesis. One example of their versatility is the Robinson annulation reaction, which leads to the formation of a substituted cyclohexenone. Treatment of a β-diketone or β-keto ester with an α,β– unsaturated ketone leads first to a Michael addition, which is followed by intramolecular aldol cyclization. Condensation reactions are also used widely in nature for the biosynthesis of such molecules as fats and steroids.

