All chemical reactions, whether in the laboratory or in living organisms, follow the same chemical rules. To understand both organic and biological chemistry, it’s necessary to know not just what occurs but also why and how chemical reactions take place. In this chapter, we’ve taken a brief look at the fundamental kinds of organic reactions, we’ve seen why reactions occur, and we’ve seen how reactions can be described.
There are four common kinds of reactions: addition reactions take place when two reactants add together to give a single product; elimination reactions take place when one reactant splits apart to give two products; substitution reactions take place when two reactants exchange parts to give two new products; and rearrangement reactions take place when one reactant undergoes a reorganization of bonds and atoms to give an isomeric product.
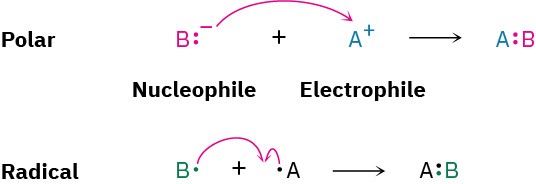
A full description of how a reaction occurs is called its mechanism. There are two general kinds of mechanisms by which most reactions take place: radical mechanisms and polar mechanisms. Polar reactions, the more common type, occur because of an attractive interaction between a nucleophilic (electron-rich) site in one molecule and an electrophilic (electron-poor) site in another molecule. A bond is formed in a polar reaction when the nucleophile donates an electron pair to the electrophile. This transfer of electrons is indicated by a curved arrow showing the direction of electron travel from the nucleophile to the electrophile. Radical reactions involve species that have an odd number of electrons. A bond is formed when each reactant donates one electron.
The energy changes that take place during reactions can be described by considering both rates (how fast the reactions occur) and equilibria (how much the reactions occur). The position of a chemical equilibrium is determined by the value of the free-energy change (ΔG) for the reaction, where ΔG = ΔH − TΔS. The enthalpy term (ΔH) corresponds to the net change in strength of chemical bonds broken and formed during the reaction; the entropy term (ΔS) corresponds to the change in the amount of molecular randomness during the reaction. Reactions that have negative values of ΔG release energy, are said to be exergonic, and have favorable equilibria. Reactions that have positive values of ΔG absorb energy, are said to be endergonic, and have unfavorable equilibria.
A reaction can be described pictorially using an energy diagram that follows the reaction course from reactants through transition state to product. The transition state is an activated complex occurring at the highest-energy point of a reaction. The amount of
energy needed by reactants to reach this high point is the activation energy, ΔG‡. The higher the activation energy, the slower the reaction.
Many reactions take place in more than one step and involve the formation of a reaction intermediate. An intermediate is a species that lies at an energy minimum between steps on the reaction curve and is formed briefly during the course of a reaction.

