As discussed in the Introduction to Organic Synthesis in Section 9.9, one of the surest ways to learn organic chemistry is to work synthesis problems. The ability to plan a successful multistep synthesis of a complex molecule requires a working knowledge of the uses and limitations of a great many organic reactions. Not only must you know which reactions to use, you must also know when to use them because the order in which reactions are carried out is often critical to the success of the overall scheme.
The ability to plan a sequence of reactions in the right order is particularly important in the synthesis of substituted aromatic rings, where the introduction of a new substituent is strongly affected by the directing effects of other substituents. Planning syntheses of substituted aromatic compounds is therefore a good way to gain confidence in using the many reactions discussed in the past few chapters.
As we said in Section 9.9, it’s usually best to work a synthesis problem backward, or retrosynthetically. Look at the target molecule and ask yourself, “What is an immediate precursor of this compound?” Choose a likely answer and continue working backward, one step at a time, until you arrive at a simple starting material. Let’s try some examples.
Worked Example 16.4 Synthesizing a Polysubstituted Benzene
How would you synthesize 4-bromo-2-nitrotoluene from benzene?
Strategy: Draw the target molecule, identify the substituents, and recall how each group can be introduced separately. Then plan retrosynthetically.

The three substituents on the ring are a bromine, a methyl group, and a nitro group. A bromine can be introduced by bromination with Br2/FeBr3, a methyl group can be introduced by Friedel–Crafts alkylation with CH3Cl/AlCl3, and a nitro group can be introduced by nitration with HNO3/H2SO4.
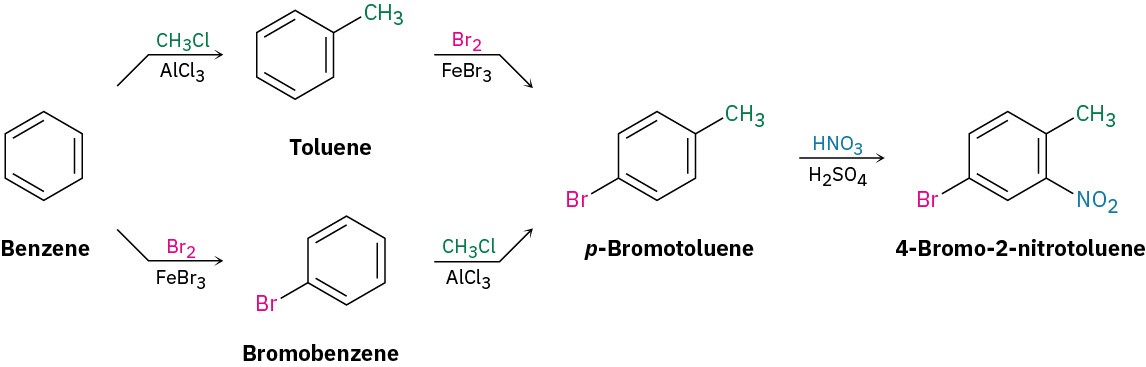
Solution: Ask yourself, “What is an immediate precursor of the target?” The final step will involve introduction of one of three groups—bromine, methyl, or nitro—so we have to consider three possibilities. Of the three, the bromination of o-nitrotoluene could be used because the activating methyl group would dominate the deactivating nitro group and direct bromination to the correct position. Unfortunately, a mixture of product isomers would be formed. A Friedel–Crafts reaction can’t be used as the final step because this reaction doesn’t work on a nitro-substituted (strongly deactivated) benzene. The best precursor of the desired product is probably p-bromotoluene, which can be nitrated ortho to the activating methyl group to give a single product.
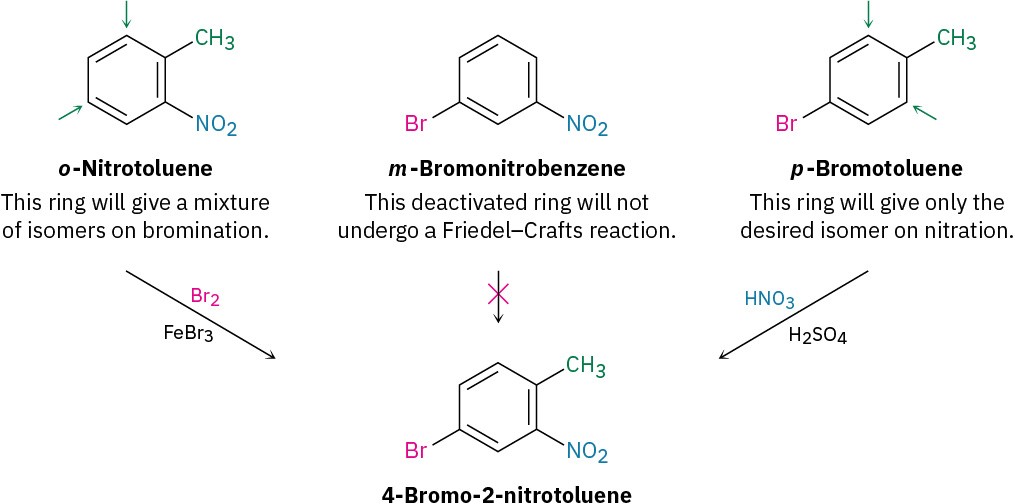
Next ask, “What is an immediate precursor of p-bromotoluene?” Perhaps toluene is an immediate precursor because the methyl group would direct bromination to the ortho and para positions. Alternatively, bromobenzene might be an immediate precursor because we could carry out a Friedel–Crafts methylation and obtain a mixture of ortho and para products. Both answers are satisfactory, although both would also lead unavoidably to a mixture of products that would have to be separated.
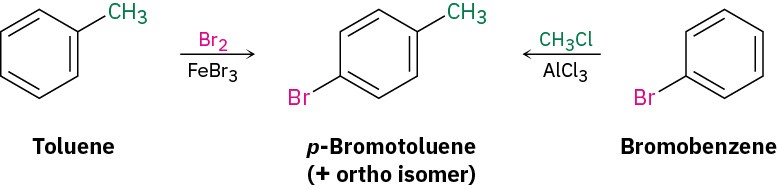
“What is an immediate precursor of toluene?” Benzene, which could be methylated in a Friedel–Crafts reaction. Alternatively, “What is an immediate precursor of bromobenzene?” Benzene, which could be brominated.The retrosynthetic analysis has provided two valid routes from benzene to 4-bromo-2-nitrotoluene.
Worked Example 16.5 Synthesizing a Polysubstituted Benzene
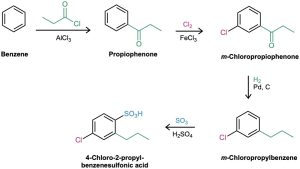
Synthesize 4-chloro-2-propylbenzenesulfonic acid from benzene.
Strategy: Draw the target molecule, identify its substituents, and recall how each of the three can be introduced. Then plan retrosynthetically.
![]()
The three substituents on the ring are a chlorine, a propyl group, and a sulfonic acid group. A chlorine can be introduced by chlorination with Cl2/FeCl3, a propyl group can be introduced by Friedel–Crafts acylation with CH3CH2COCl/AlCl3 followed by reduction with H2/Pd, and a sulfonic acid group can be introduced by sulfonation with SO3/H2SO4.
Solution: “What is an immediate precursor of the target?” The final step will involve introduction of one of three groups—chlorine, propyl, or sulfonic acid—so we have to consider three possibilities. Of the three, the chlorination of o-propylbenzenesulfonic acid can’t be used because the reaction would occur at the wrong position. Similarly, a Friedel–Crafts reaction can’t be used as the final step because this reaction doesn’t work on sulfonic-acid- substituted (strongly deactivated) benzenes. Thus, the immediate precursor of the desired product is probably m-chloropropylbenzene, which can be sulfonated to give a mixture of product isomers that must then be separated.

“What is an immediate precursor of m-chloropropylbenzene?” Because the two substituents have a meta relationship, the first substituent placed on the ring must be a meta director so that the second substitution will take place at the proper position.Furthermore, because primary alkyl groups such as propyl can’t be introduced directly by Friedel–Crafts alkylation, the precursor of m-chloropropylbenzene is probably m-chloropropiophenone, which could be catalytically reduced.
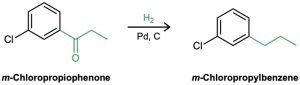
“What is an immediate precursor of m-chloropropiophenone?” Propiophenone, which could be chlorinated in the meta position.
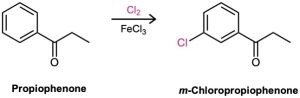
“What is an immediate precursor of propiophenone?” Benzene, which could undergo Friedel–Crafts acylation with propanoyl chloride and AlCl3.
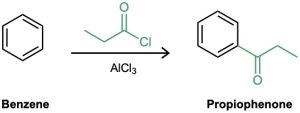
The final synthesis is a four-step route from benzene:
Planning an organic synthesis has been compared with playing chess. There are no tricks; all that’s required is a knowledge of the allowable moves (the organic reactions) and the discipline to plan ahead, carefully evaluating the consequences of each move. Practicing may not be easy, but it’s a great way to learn organic chemistry.
Problem 16-22
How might you synthesize the following substances from benzene?
(a) m-Chloronitrobenzene
(b) m-Chloroethylbenzene
(c) 4-Chloro-1-nitro-2-propylbenzene
(d) 3-Bromo-2-methylbenzenesulfonic acid
Problem 16-23
In planning a synthesis, it’s as important to know what not to do as to know what to do. As written, the following reaction schemes have flaws in them. What is wrong with each?
(a)
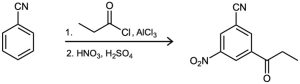
(b)
![]()
Additional Problems 16
Visualizing Chemistry
Problem 16-24
Draw the product from reaction of each of the following substances with (1) Br2, FeBr3 and (2) CH3COCl, AlCl3.
(a)
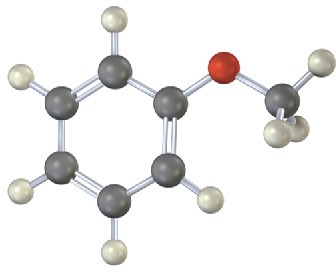
(b)
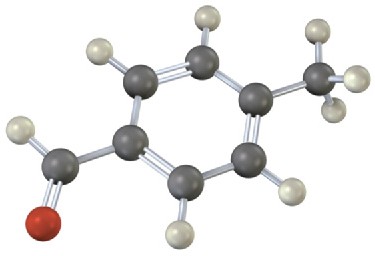
Problem 16-25
The following molecular model of a dimethyl-substituted biphenyl represents the lowest- energy conformation of the molecule. Why are the two benzene rings tilted at a 63° angle to each other rather than being in the same plane so that their p orbitals overlap? Why doesn’t complete rotation around the single bond joining the two rings occur?
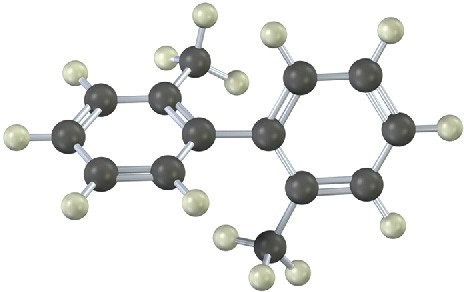
Problem 16-26
How would you synthesize the following compound starting from benzene? More than one step is needed.
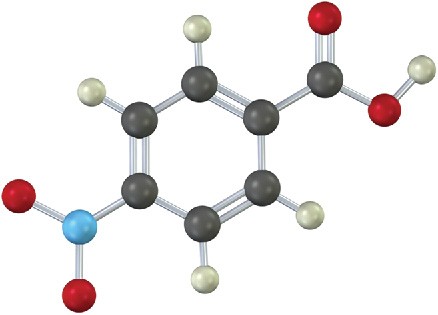
Problem 16-27
The following compound can’t be synthesized using the methods discussed in this chapter. Why not?
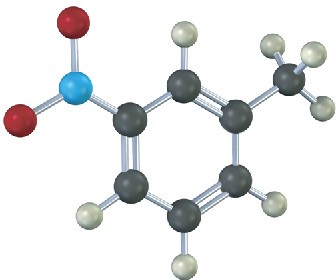
Mechanism Problems
Mechanisms of Electrophilic Substitutions
Problem 16-28
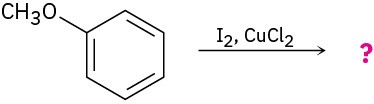
Aromatic iodination can be carried out with a number of reagents, including iodine monochloride, ICl. What is the direction of polarization of ICl? Propose a mechanism for the iodination of an aromatic ring with ICl.
Problem 16-29
The sulfonation of an aromatic ring with SO3 and H2SO4 is reversible. That is, heating benzenesulfonic acid with H2SO4 yields benzene. Show the mechanism of the desulfonation reaction. What is the electrophile?
Problem 16-30
The carbocation electrophile in a Friedel–Crafts reaction can be generated by an alternate means than reaction of an alkyl chloride with AlCl3. For example, reaction of benzene with 2-methylpropene in the presence of H3PO4 yields tert-butylbenzene. Propose a mechanism for this reaction.
Problem 16-31
The N,N,N-trimethylammonium group, – 𝑁(CH3)3, is one of the few groups that is a meta-directing deactivator yet has no electron- withdrawing resonance effect. Explain.
Problem 16-32
The nitroso group, –N=O, is one of the few nonhalogens that is an ortho- and para-directing deactivator. Explain this behavior by drawing resonance structures of the carbocation intermediates in ortho, meta, and para electrophilic reaction on nitrosobenzene, C6H5N=O.
Problem 16-33
Triphenylmethane can be prepared by reaction of benzene and chloroform in the presence of AlCl3. Propose a mechanism for the reaction.
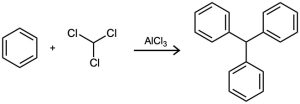
Problem 16-34
Using resonance structures of the intermediates, explain why bromination of biphenyl occurs at ortho and para positions rather than at meta.
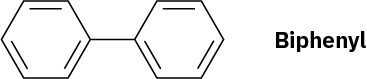
Problem 16-35
Benzene and alkyl-substituted benzenes can be hydroxylated by reaction with H2O2 in the presence of an acidic catalyst. What is the structure of the reactive electrophile? Propose a mechanism for the reaction.
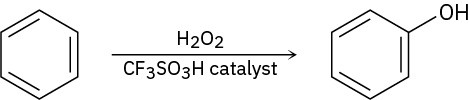
Additional Mechanism Practice
Problem 16-36
Addition of HBr to 1-phenylpropene yields only (1-bromopropyl)benzene. Propose a mechanism for the reaction, and explain why none of the other regioisomer is produced.
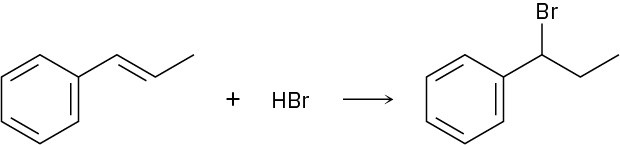
Problem 16-37
Hexachlorophene, a substance used in the manufacture of germicidal soaps, is prepared by reaction of 2,4,5-trichlorophenol with formaldehyde in the presence of concentrated sulfuric acid. Propose a mechanism for the reaction.
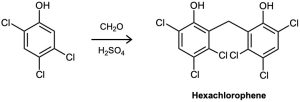
Problem 16-38
Benzenediazonium carboxylate decomposes when heated to yield N2, CO2, and a reactive substance that can’t be isolated. When benzene-diazonium carboxylate is heated in the presence of furan, the following reaction is observed:
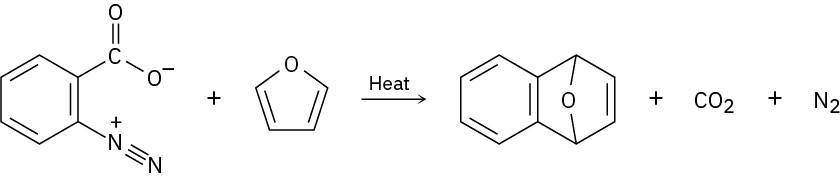
What intermediate is involved in this reaction? Propose a mechanism for its formation.
Problem 16-39
4-Chloropyridine undergoes reaction with dimethylamine to yield 4-dimethylaminopyridine. Propose a mechanism for the reaction.
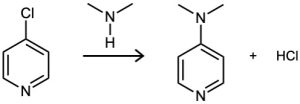
Problem 16-40
Propose a mechanism to account for the following reaction:
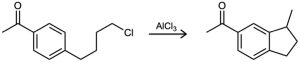
Problem 16-41
In the Gatterman–Koch reaction, a formyl group (–CHO) is introduced directly onto a benzene ring. For example, reaction of toluene with CO and HCl in the presence of mixed CuCl/AlCl3 gives p-methylbenzaldehyde. Propose a mechanism.
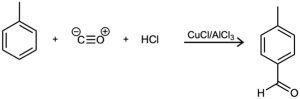
Problem 16-42
Treatment of p–tert-butylphenol with a strong acid such as H2SO4 yields phenol and 2-methylpropene. Propose a mechanism.
Problem 16-43
Benzyl bromide is converted into benzaldehyde by heating in dimethyl sulfoxide. Propose a structure for the intermediate, and show the mechanisms of the two steps in the reaction.
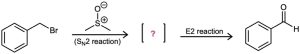
Problem 16-44
Propose a mechanism for the Smiles rearrangement below.
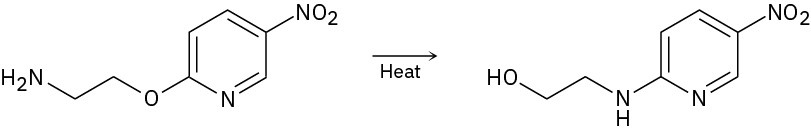
Problem 16-45
Because of their conjugation, azo dyes are highly colored compounds and the major artificial color source for textiles and food. Azo dyes are produced by the reaction of aryl diazonium salts with a second aromatic compound. In the product, the aromatic rings are linked by a diazo bridge (–N=N–). From the reactants provided, propose a structure for each azo dye and draw the electron-pushing mechanism.
(a)
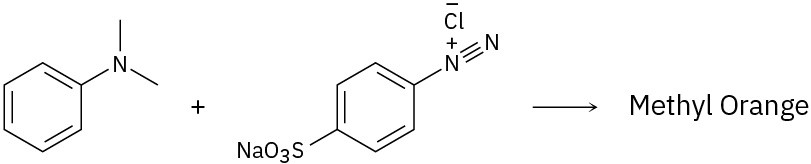
(b)
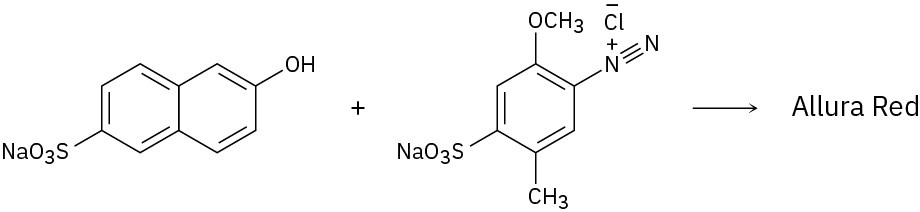
(c)
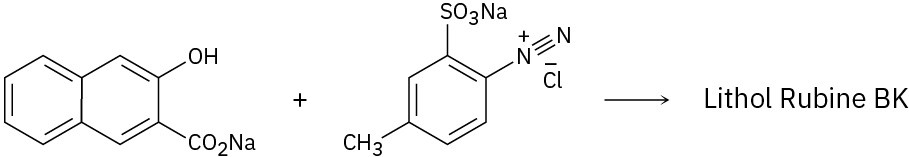
Reactivity and Orientation of Electrophilic Substitutions
Problem 16-46
Identify each of the following groups as an activator or deactivator and as an o,p-director or m-director:
(a)
![]()
(b)
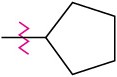
(c)
![]()
(d)
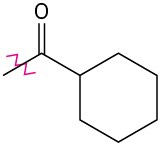
Problem 16-47
Predict the major product(s) of nitration of the following substances. Which react faster than benzene, and which slower?
(a) Bromobenzene
(b) Benzonitrile
(c) Benzoic acid
(d) Nitrobenzene
(e) Benzenesulfonic acid
(f) Ethoxybenzene
Problem 16-48
Rank the compounds in each group according to their reactivity toward electrophilic substitution.
(a) Chlorobenzene, o-dichlorobenzene, benzene
(b) p-Bromonitrobenzene, nitrobenzene, phenol
(c) Fluorobenzene, benzaldehyde, o-xylene
(d) Benzonitrile, p-methylbenzonitrile, p-methoxybenzonitrile
Problem 16-49
Predict the major monoalkylation products you would expect to obtain from reaction of the following substances with chloromethane and AlCl3:
(a) Bromobenzene
(b) m-Bromophenol
(c) p-Chloroaniline
(d) 2,4-Dichloronitrobenzene
(e) 2,4-Dichlorophenol
(f) Benzoic acid
(g) p-Methylbenzenesulfonic acid
(h) 2,5-Dibromotoluene
Problem 16-50
Name and draw the major product(s) of electrophilic chlorination of the following compounds:
(a) m-Nitrophenol
(b) Xylene
(c) Nitrobenzoic acid
(d) p-Bromobenzenesulfonic acid
Problem 16-51
Predict the major product(s) you would obtain from sulfonation of the following compounds:
(a) Fluorobenzene
(b) m-Bromophenol
(c) m-Dichlorobenzene
(d) 2,4-Dibromophenol
Problem 16-52
Rank the following aromatic compounds in the expected order of their reactivity toward Friedel–Crafts alkylation. Which compounds are unreactive?
(a) Bromobenzene
(b) Toluene
(c) Phenol
(d) Aniline
(e) Nitrobenzene
(f) p-Bromotoluene
Problem 16-53
What product(s) would you expect to obtain from the following reactions:
(a)
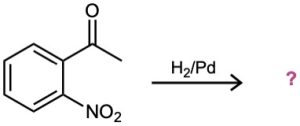
(b)
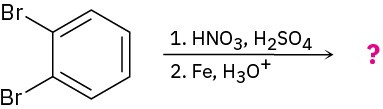
(c)
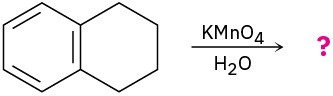
(d)
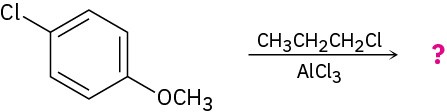
Problem 16-54
Predict the major product(s) of the following reactions:
(a)
![]()
(b)
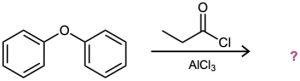
(c)
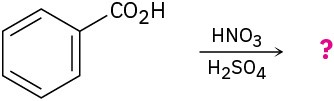
(d)
Organic Synthesis
Problem 16-55
How would you synthesize the following substances starting from benzene or phenol? Assume that ortho- and para-substitution products can be separated.
(a) o-Bromobenzoic acid
(b) p-Methoxytoluene
(c) 2,4,6-Trinitrobenzoic acid
(d) m-Bromoaniline
Problem 16-56
Starting with benzene as your only source of aromatic compounds, how would you synthesize the following substances? Assume that you can separate ortho and para isomers if necessary.
(a) p-Chloroacetophenone
(b) m-Bromonitrobenzene
(c) o-Bromobenzenesulfonic acid
(d) m-Chlorobenzenesulfonic acid
Problem 16-57
Starting with either benzene or toluene, how would you synthesize the following substances: Assume that ortho and para isomers can be separated.
(a) 2-Bromo-4-nitrotoluene
(b) 1,3,5-Trinitrobenzene
(c) 2,4,6-Tribromoaniline
(d) m-Fluorobenzoic acid
Problem 16-58
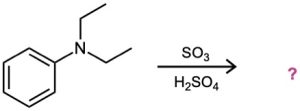
As written, the following syntheses have flaws. What is wrong with each?
(a)
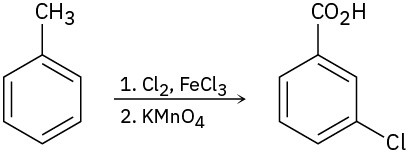
(b)
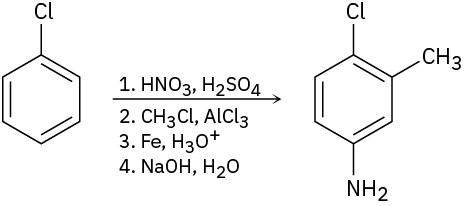
(c)
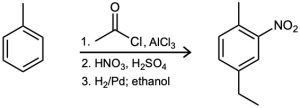
General Problems
Problem 16-59
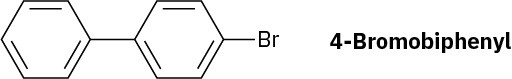
At what position and on what ring do you expect nitration of 4-bromo-biphenyl to occur? Explain, using resonance structures of the potential intermediates.
Problem 16-60
Electrophilic substitution on 3-phenylpropanenitrile occurs at the ortho and para positions, but reaction with 3-phenylpropenenitrile occurs at the meta position. Explain, using resonance structures of the intermediates.
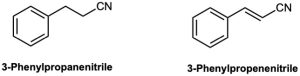
Problem 16-61
At what position, and on what ring, would you expect the following substances to undergo electrophilic substitution?
(a)
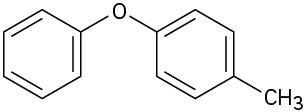
(b)
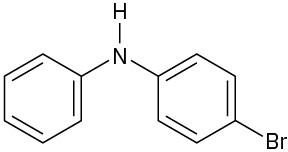
(c)
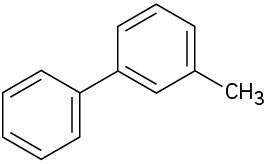
(d)
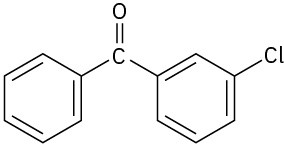
Problem 16-62
At what position, and on what ring, would you expect bromination of benzanilide to occur? Explain by drawing resonance structures of the intermediates.
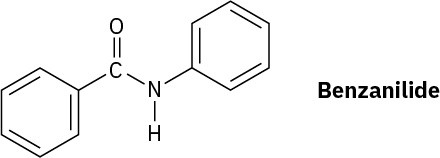
Problem 16-63
Would you expect the Friedel–Crafts reaction of benzene with (R)-2-chlorobutane to yield optically active or racemic product? Explain.
Problem 16-64
How would you synthesize the following substances starting from benzene?
(a)
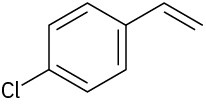
(b)
![]()
(c)
![]()
Problem 16-65
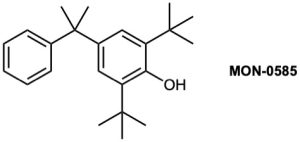
The compound MON-0585 is a nontoxic, biodegradable larvicide that is highly selective against mosquito larvae. Synthesize MON-0585 using either benzene or phenol as a source of the aromatic rings.
Problem 16-66
Phenylboronic acid, C6H5B(OH)2, is nitrated to give 15% ortho- substitution product and 85% meta. Explain the meta-directing effect of the –B(OH)2 group.
Problem 16-67
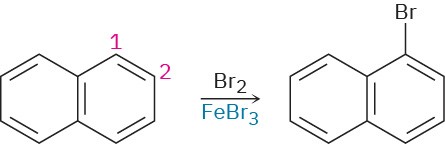
Draw resonance structures of the intermediate carbocations in the bromination of naphthalene, and account for the fact that naphthalene undergoes electrophilic substitution at C1 rather than C2.
Problem 16-68
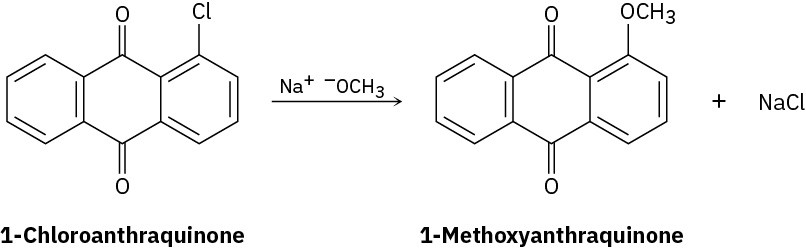
Propose a mechanism for the reaction of 1-chloroanthraquinone with methoxide ion to give the substitution product 1-methoxyanthraquinone. Use curved arrows to show the electron flow in each step.
Problem 16-69
p-Bromotoluene reacts with potassium amide to give a mixture of m– and p-methylaniline. Explain.
Problem 16-70
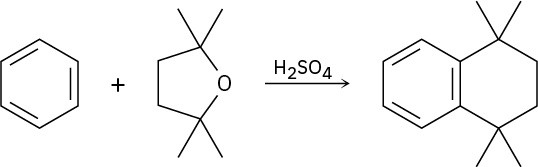
Propose a mechanism to account for the reaction of benzene with 2,2,5,5-tetramethyltetrahydrofuran.
Problem 16-71
How would you synthesize the following compounds from benzene? Assume that ortho and para isomers can be separated.
(a)
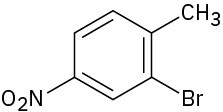
(b)

Problem 16-72
You know the mechanism of HBr addition to alkenes, and you know the effects of various substituent groups on aromatic substitution. Use this knowledge to predict which of the following two alkenes reacts faster with HBr. Explain your answer by drawing resonance structures of the carbocation intermediates.

Problem 16-73
Use your knowledge of directing effects, along with the following data, to deduce the directions of the dipole moments in aniline, bromobenzene, and bromoaniline.
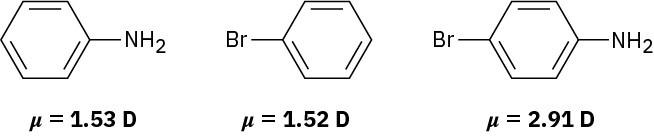
Problem 16-74
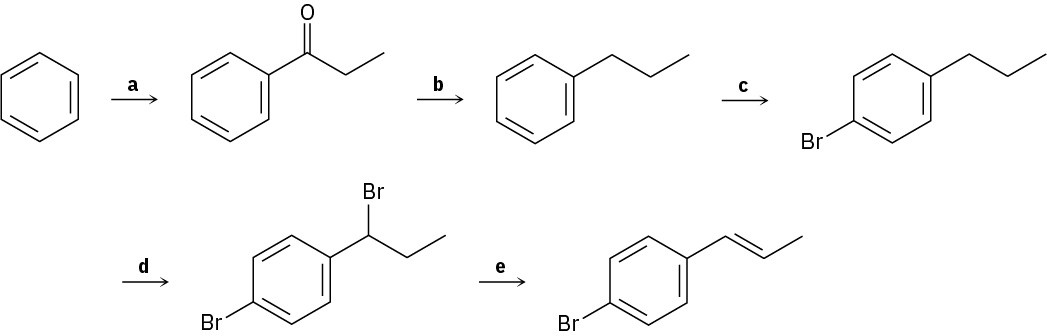
Identify the reagents represented by the letters a–e in the following scheme:
Problem 16-75
Phenols (ArOH) are relatively acidic, and the presence of a substituent group on the aromatic ring has a large effect. The pKa of unsubstituted phenol, for example, is 9.89, while that of p-nitrophenol is 7.15. Draw resonance structures of the corresponding phenoxide anions and explain the data.
Problem 16-76
Would you expect p-methylphenol to be more acidic or less acidic than unsubstituted phenol? Explain. (See Problem 16-75.)
Problem 16-77
Predict the product(s) for each of the following reactions. In each case, draw the resonance forms of the intermediate to explain the observed regiochemistry.
(a)
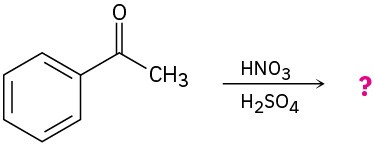
(b)
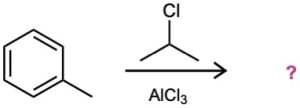
(c)
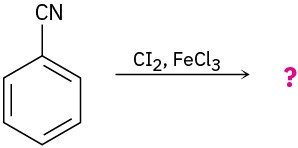
(d)