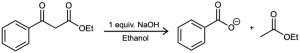
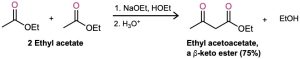
Esters, like aldehydes and ketones, are weakly acidic. When an ester with an α hydrogen is treated with 1 equivalent of a base such as sodium ethoxide, a reversible carbonyl condensation reaction occurs to yield a β-keto ester. For instance, ethyl acetate yields ethyl acetoacetate on base treatment. This reaction between two ester molecules is known as the Claisen condensation reaction. (We’ll use ethyl esters, abbreviated “Et,” for consistency, but other esters will also work.)
The mechanism of the Claisen condensation is similar to that of the aldol condensation and involves the nucleophilic addition of an ester enolate ion to the carbonyl group of a second ester molecule (Figure 23.5). The only difference between the aldol condensation of an aldehyde or ketone and the Claisen condensation of an ester involves the fate of the initially formed tetrahedral intermediate. The tetrahedral intermediate in the aldol reaction is protonated to give an alcohol product—exactly the behavior previously seen for aldehydes and ketones (Section 19.4). The tetrahedral intermediate in the Claisen reaction, however, expels an alkoxide leaving group to yield an acyl substitution product—as previously seen for esters (Section 21.6).
Figure 23.5 Mechanism of the Claisen condensation reaction.
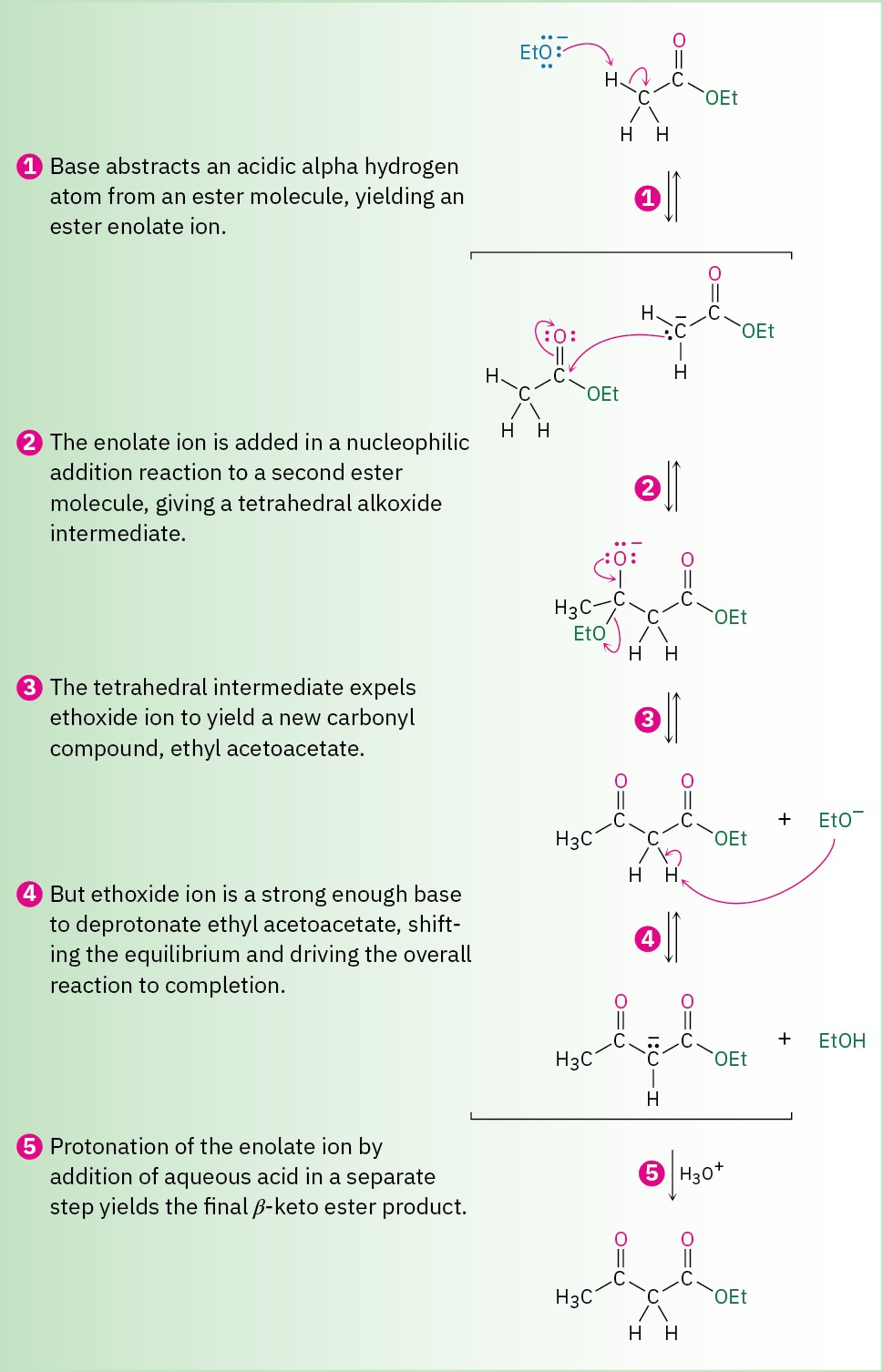
If the starting ester has more than one acidic α hydrogen, the product β-keto ester has a highly acidic, doubly activated hydrogen atom that can be abstracted by base. This deprotonation of the product requires the use of a full equivalent of base rather than a catalytic amount. Furthermore, the deprotonation serves to drive the equilibrium completely to the product side so that high yields are usually obtained in Claisen condensations.
Worked Example 23.3 – Predicting the Product of a Claisen Condensation Reaction
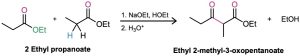
What product would you obtain from Claisen condensation of ethyl propanoate?
Strategy: The Claisen condensation of an ester results in loss of one molecule of alcohol and formation of a product in which an acyl group of one reactant bonds to the α carbon of the second reactant. The product is a β-keto ester.
Solution:
Problem 23-11
Show the products you would expect to obtain by Claisen condensation of the following esters:
(a) (CH3)2CHCH2CO2Et
(b) ethyl phenylacetate
(c) ethyl cyclohexylacetate
Problem 23-12
As shown in Figure 23.5, the Claisen reaction is reversible. That is, a β-keto ester can be cleaved by base into two fragments. Using curved arrows to indicate electron flow, show the mechanism by which this cleavage occurs.