Humans need to obtain eight monosaccharides for proper functioning. Although all eight can be biosynthesized from simpler precursors if necessary, it’s more energetically efficient to obtain them from the diet. The eight are L-fucose (6-deoxy-L-galactose), D-galactose, D-glucose, D-mannose, N-acetyl-D-glucosamine, N-acetyl-D-galactosamine, D-xylose, and N-acetyl-D-neuraminic acid (Figure 25.10). All are used for the synthesis of the glycoconjugate components of cell membranes, and glucose is also the body’s primary source of energy.
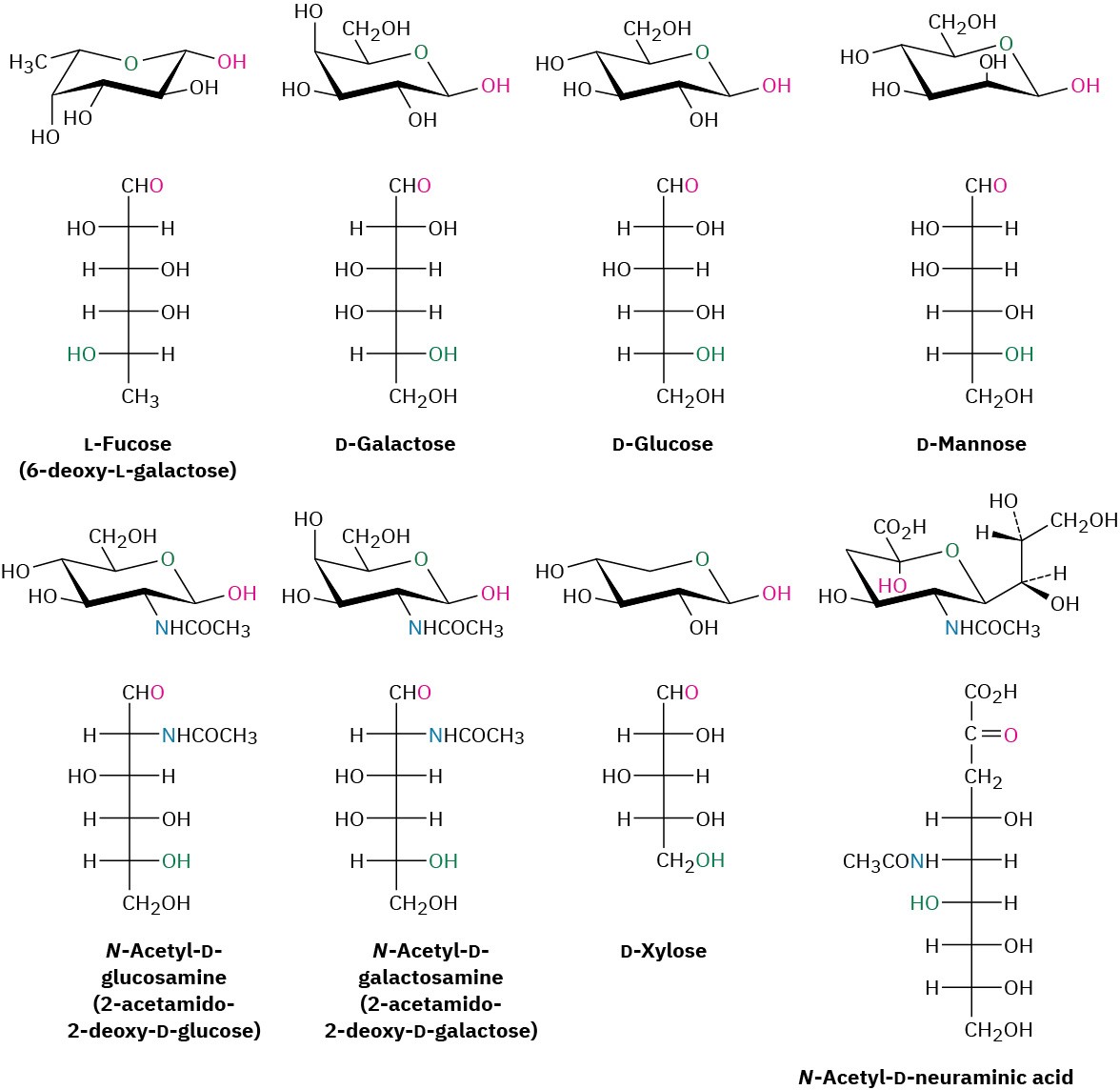
Figure 25.10 Structures of the eight monosaccharides essential to humans.
Of the eight essential monosaccharides, galactose, glucose, and mannose are simple aldohexoses, while xylose is an aldopentose. Fucose is a deoxy sugar, meaning that it has an oxygen atom “missing.” That is, an –OH group (the one at C6) is replaced by an –H. N-Acetylglucosamine and N-acetylgalactosamine are amide derivatives of amino sugars in which an –OH (the one at C2) is replaced by an –NH2 group. N-Acetylneuraminic acid is the parent compound of the sialic acids, a group of more than 30 compounds with different modifications, including various oxidations, acetylations, sulfations, and methylations. Note that neuraminic acid has nine carbons and is an aldol reaction product of N-acetylmannosamine with pyruvate (CH3COCO2–). Neuraminic acid is crucial to the mechanism by which an influenza virus spreads.
All the essential monosaccharides arise from glucose, by the conversions summarized in Figure 25.11. We’ll not look specifically at these conversions, but might note that Problems 25-30 through 25-32 and 25-35 at the end of the chapter lead you through several of the biosynthetic pathways.
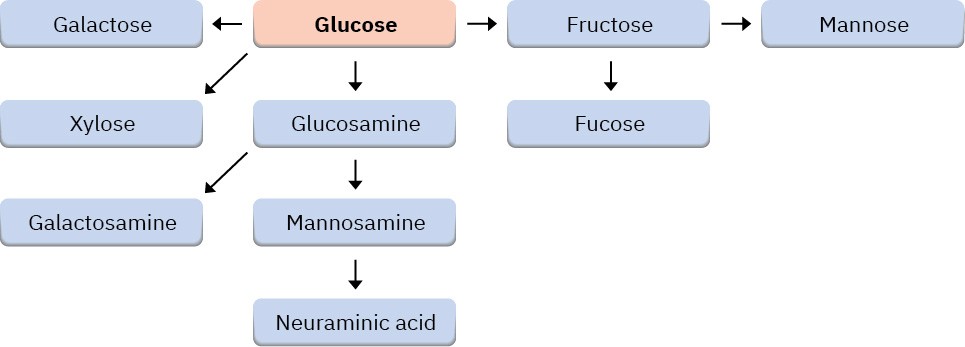
Figure 25.11 An overview of biosynthetic pathways for the eight essential monosaccharides.
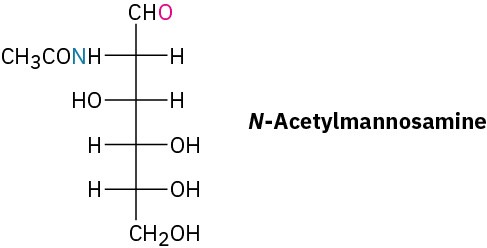
Problem 25-24
Show how neuraminic acid can arise by an aldol reaction of N-acetylmannosamine with pyruvate (CH3COCO2–).