A molecule that is not identical to its mirror image is said to be chiral (ky-ral, from the Greek cheir, meaning “hand”). You cannot take a chiral molecule and its enantiomer and place one on the other so that all atoms coincide.
How can you predict whether a given molecule is or is not chiral? A molecule is not chiral if it has a plane of symmetry. A plane of symmetry is a plane that cuts through the middle of a molecule (or any object) in such a way that one half of the molecule or object is a mirror image of the other half. A coffee mug, for example, has a plane of symmetry. If you were to cut the coffee mug in half from top to bottom, one half would be a mirror image of the other half. A hand, however, does not have a plane of symmetry. One “half” of a hand is not a mirror image of the other half (Figure 5.4).
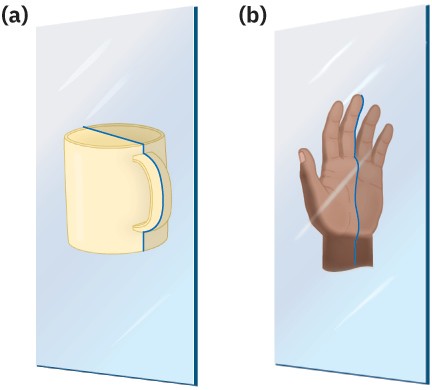
Figure 5.4 The meaning of symmetry plane. (a) An object like the coffee mug has a symmetry plane cutting through it so that right and left halves are mirror images. (b) An object like a hand has no symmetry plane; the right “half” of a hand is not a mirror image of the left half.
A molecule that has a plane of symmetry in any conformation must be identical to its mirror image and must be nonchiral, or achiral. Thus, propanoic acid, CH3CH2CO2H, has a plane of symmetry when lined up as shown in Figure 5.5 and is achiral, while lactic acid, CH3CH(OH)CO2H, has no plane of symmetry in any conformation and is chiral.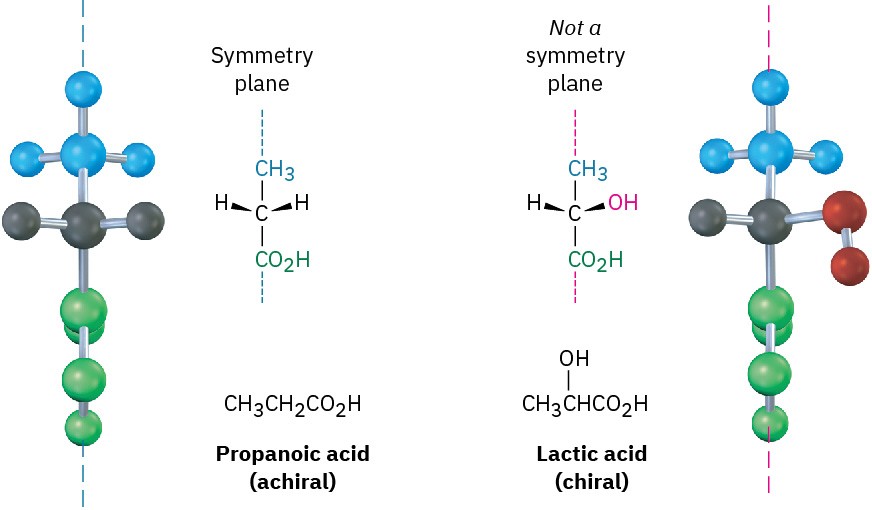
Figure 5.5 The achiral propanoic acid molecule versus the chiral lactic acid molecule. Propanoic acid has a plane of symmetry that makes one side of the molecule a mirror image of the other. Lactic acid has no such symmetry plane.
The most common, although not the only, cause of chirality in organic molecules is the presence of a tetrahedral carbon atom bonded to four different groups—for example, the central carbon atom in lactic acid. Such carbons are referred to as stereocenters, although other terms such as asymmetric center or chirality center are also used. Note that chirality is a property of the entire molecule, whereas a stereocenter is the cause of chirality.
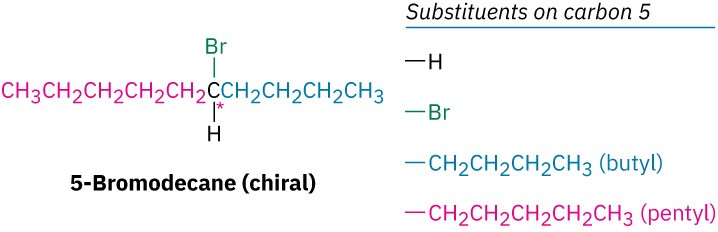
Detecting a stereocenter in a complex molecule takes practice because it’s not always immediately apparent whether four different groups are bonded to a given carbon. The differences don’t necessarily appear right next to the stereocenter. For example, 5- bromodecane is a chiral molecule because four different groups are bonded to C5, the stereocenter (marked with an asterisk). A butyl substituent is similar to a pentyl substituent, but it isn’t identical. The difference isn’t apparent until looking four carbon atoms away from the stereocenter, but there’s still a difference.
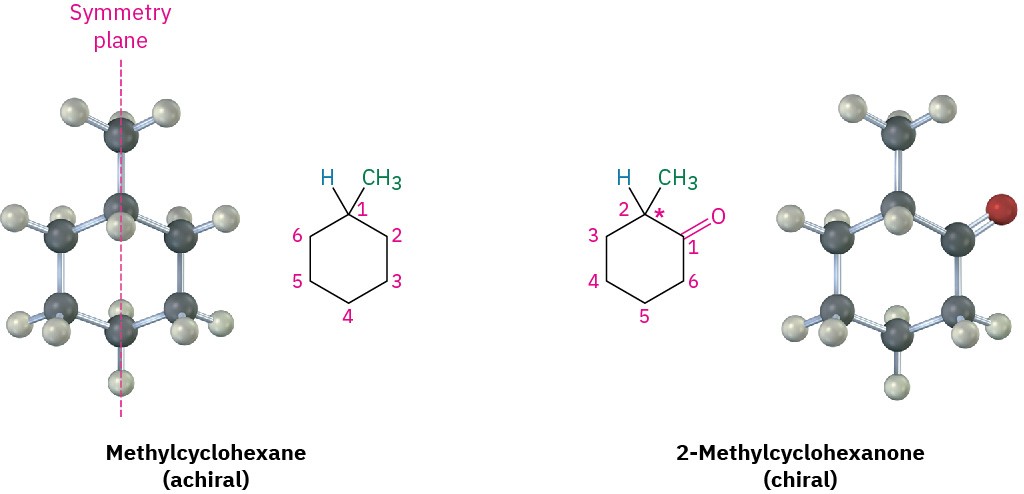
As other possible examples, look at methylcyclohexane and 2-methylcyclohexanone. Methylcyclohexane is achiral because no carbon atom in the molecule is bonded to four different groups. One way of reaching this conclusion is to realize that methylcyclohexane has a symmetry plane, which passes through the methyl group and through C1 and C4 of the ring.
The situation is different for 2-methylcyclohexanone. 2-Methylcyclohexanone has no symmetry plane and is chiral because its C2 is bonded to four different groups: a −CH3 group, an −H atom, a −COCH2− ring bond (C1), and a −CH2CH2− ring bond (C3).
Several more examples of chiral molecules are shown below. Check for yourself that the labeled carbons are stereocenters. You might note that carbons in −CH2−, −CH3, C═O, C═C, and C≡C groups can’t be stereocenters. (Why not?)
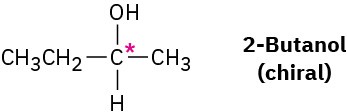
Worked Example 5.1
Drawing the Three-Dimensional Structure of a Chiral Molecule
Draw the structure of a chiral alcohol.
Strategy – An alcohol is a compound that contains the −OH functional group. To make an alcohol chiral, we need to have four different groups bonded to a single carbon atom, say −H, −OH,−CH3, and −CH2CH3.
Solution
Problem 5-1
Which of the following objects are chiral?
(a) Soda can (b) Screwdriver (c) Screw (d) Shoe
Problem 5-2
Which of the following molecules are chiral? Identify the stereocenter(s) in each.
(a)
(b) 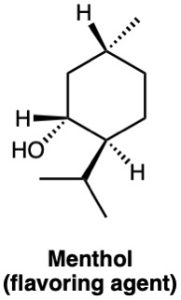
(c) 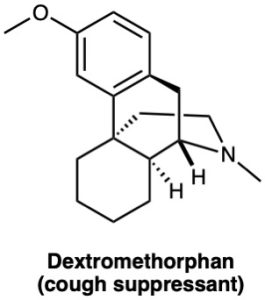
Problem 5-3
Alanine, an amino acid found in proteins, is chiral. Draw the two enantiomers of alanine using the standard convention of solid, wedged, and dashed lines.
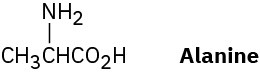
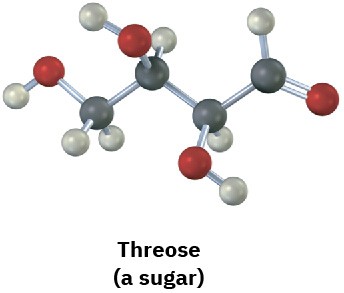
Problem 5-4
Identify the stereocenters in the following molecules (gray = H, black = C, red = O, green = Cl, yellow-green = F):
(a)
(b)