Most nucleophilic substitutions take place by the SN2 pathway just discussed. The reaction is favored when carried out with an unhindered substrate and a negatively charged nucleophile in a polar aprotic solvent, but is disfavored when carried out with a hindered substrate and a neutral nucleophile in a protic solvent. You might therefore expect the reaction of a tertiary substrate (hindered) with water (neutral, protic) to be among the slowest of substitution reactions. Remarkably, however, the opposite is true. The reaction of the tertiary halide 2-bromo-2-methylpropane (CH3)3CBr with H2O to give the alcohol 2-methyl-2-propanol is more than 1 million times faster than the corresponding reaction of CH3Br to give methanol.
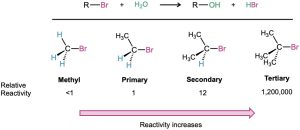
What’s going on here? A nucleophilic substitution reaction is occurring—a hydroxyl group is replacing a halogen—yet the reactivity order seems backward. These reactions can’t be taking place by the SN2 mechanism we’ve been discussing, so we must therefore conclude that they are occurring by an alternative substitution mechanism. This alternative mechanism is called the SN1 reaction, for substitution, nucleophilic, unimolecular.
In contrast to the SN2 reaction of CH3Br with OH–, the SN1 reaction of (CH3)3CBr with H2O has a rate that depends only on the alkyl halide concentration and is independent of the H2O concentration. In other words, the process is a first-order reaction; the concentration of the nucleophile does not appear in the rate equation.

To explain this result, we need to know more about kinetics measurements. Many organic reactions occur in several steps, one of which usually has a higher-energy transition state than the others and is therefore slower. We call this step with the highest transition-state energy the rate-limiting step, or rate-determining step. No reaction can proceed faster than its rate-limiting step, which acts as a kind of traffic jam, or bottleneck. In the SN1 reaction of (CH3)3CBr with H2O, the fact that the nucleophile concentration does not appear in the first-order rate equation means that it is not involved in the rate-limiting step and must therefore be involved in some other, non-rate-limiting step. The mechanism shown in Figure 11.9 accounts for these observations.
Figure 11.9 MECHANISM
The mechanism of the SN1 reaction of 2-bromo-2-methylpropane with H2O involves three steps. Step 1—the spontaneous, unimolecular dissociation of the alkyl bromide to yield a carbocation—is rate-limiting.
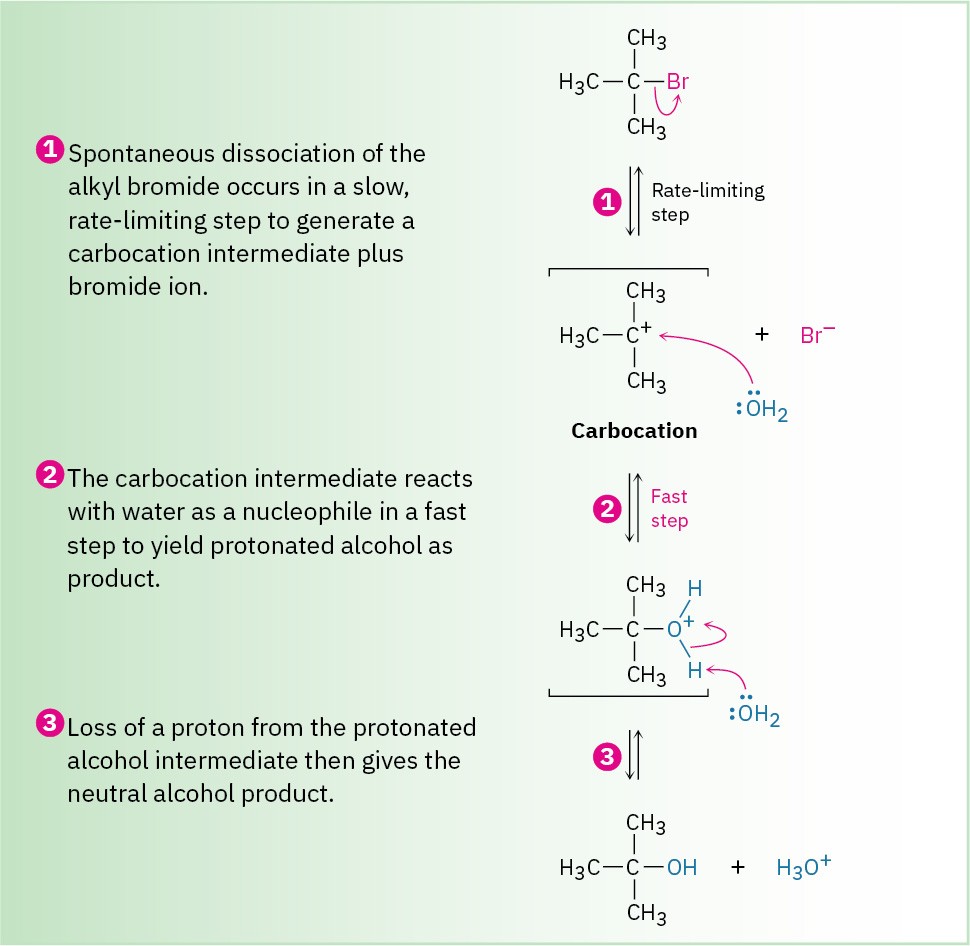
Unlike what occurs in an SN2 reaction, where the leaving group is displaced while the incoming nucleophile approaches, an SN1 reaction takes place by loss of the leaving group before the nucleophile approaches. 2-Bromo-2-methylpropane spontaneously dissociates to the tert-butyl carbocation (CH3)3C+, plus Br– in a slow, rate-limiting step, and the intermediate carbocation is then immediately trapped by the nucleophile water in a faster second step. Thus, water is not a reactant in the step whose rate is measured. The energy diagram is shown in Figure 11.10.
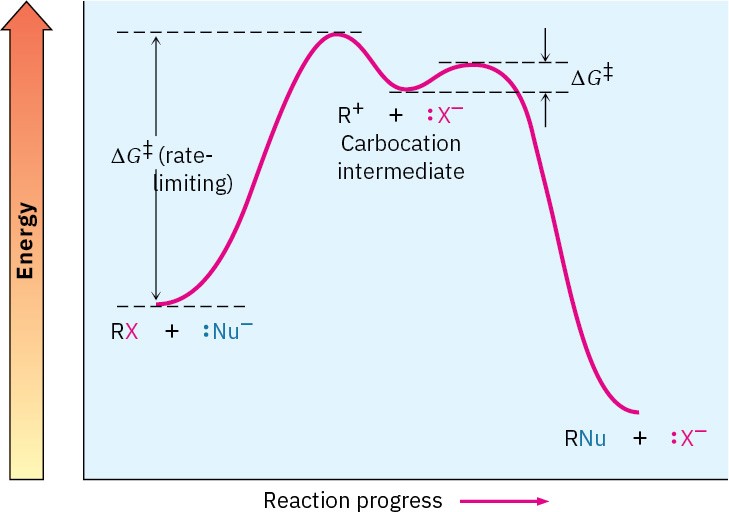
Figure 11.10 An energy diagram for an SN1 reaction. The rate-limiting step is the spontaneous dissociation of the alkyl halide to give a carbocation intermediate. Reaction of the carbocation with a nucleophile then occurs in a second, faster step.
Because an SN1 reaction occurs through a carbocation intermediate, its stereochemical outcome is different from that of an SN2 reaction. Carbocations, as we’ve seen, are planar, sp2-hybridized, and achiral. Thus, if we carry out an SN1 reaction on one enantiomer of a chiral reactant and go through an achiral carbocation intermediate, the product loses its optical activity (Section 8.12). That is, the symmetrical intermediate carbocation can react with a nucleophile equally well from either side, leading to a racemic, 50 : 50 mixture of enantiomers (Figure 11.11).
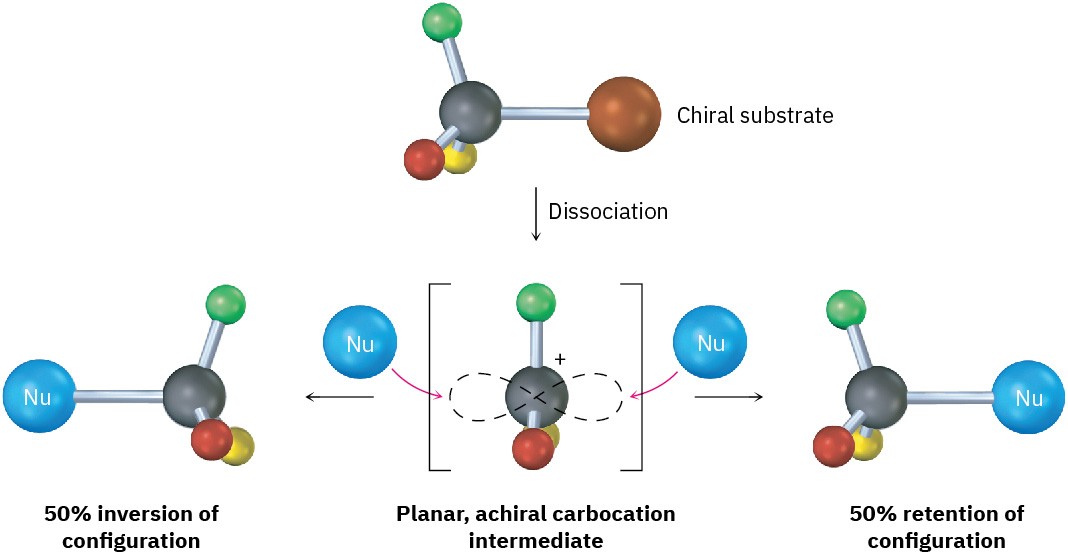
Figure 11.11 Stereochemistry of the SN1 reaction. Because the reaction goes through an achiral intermediate, an enantiomerically pure reactant gives an optically inactive racemic product.
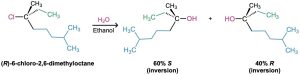
The conclusion that SN1 reactions on enantiomerically pure substrates should give racemic products is nearly, but not exactly, what is found. In fact, few SN1 displacements occur with complete racemization. Most give a minor (0–20%) excess of inversion. The reaction of (R)-6-chloro-2,6-dimethyloctane with H2O, for example, leads to an alcohol product that is approximately 80% racemized and 20% inverted (equivalent to 40% R + 60% S).
This lack of complete racemization in SN1 reactions is due to the fact that ion pairs are involved. According to this explanation, first proposed by Saul Winstein at UCLA, dissociation of the substrate occurs to give a structure in which the two ions are still loosely associated and in which the carbocation is effectively shielded from reaction on one side by the departing anion. If a certain amount of substitution occurs before the two ions fully diffuse apart, then a net inversion of configuration will be observed (Figure 11.12).
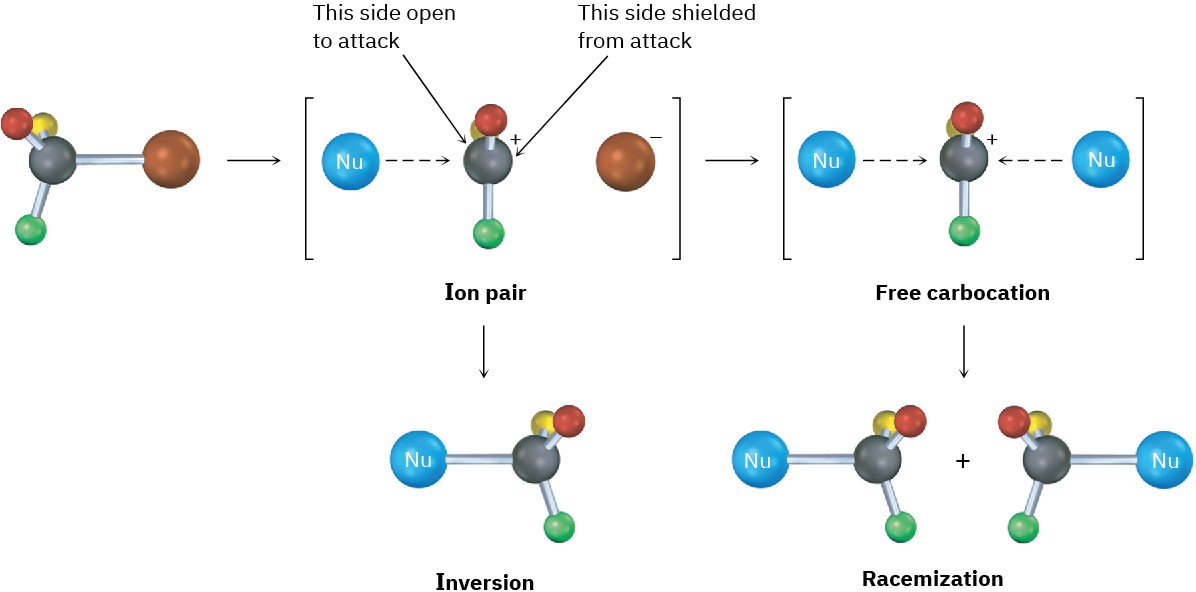
Figure 11.12 Ion pairs in an SN1 reaction. The leaving group shields one side of the carbocation intermediate from reaction with the nucleophile, thereby leading to some inversion of configuration rather than complete racemization.
Problem 11-8
What product(s) would you expect from reaction of (S)-3-chloro-3-methyloctane with acetic acid? Show the stereochemistry of both reactant and product.
Problem 11-9
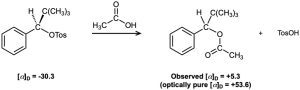
Among the many examples of SN1 reactions that occur with incomplete racemization, the optically pure tosylate of 2,2-dimethyl-1-phenyl-1-propanol ([α]D = –30.3) gives the corresponding acetate ([α]D = +5.3) when heated in acetic acid. If complete inversion had occurred, the optically pure acetate would have had [α]D = +53.6. What percentage racemization and what percentage inversion occurred in this reaction?
Problem 11-10
Assign configuration to the following substrate, and show the stereochemistry and identity of the product you would obtain by SN1 reaction with water (reddish brown = Br):