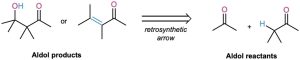
The aldol reaction yields either a β-hydroxy aldehyde/ketone or an α,β-unsaturated aldehyde/ketone, depending on the experimental conditions. By learning how to work backward, it’s possible to predict when the aldol reaction might be useful in synthesis. Whenever the target molecule contains either a β-hydroxy aldehyde/ketone or a conjugated enone functional group, it might come from an aldol reaction.
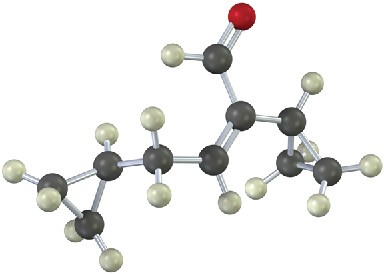
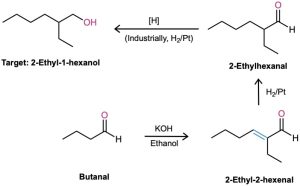
We can extend this kind of reasoning even further by imagining that subsequent transformations might be carried out on the aldol products. For example, a saturated ketone might be prepared by catalytic hydrogenation of the enone product. A good example can be found in the industrial preparation of 2-ethyl-1-hexanol, an alcohol used in the synthesis of plasticizers for polymers. Although 2-ethyl-1-hexanol bears little resemblance to an aldol product at first glance, it is in fact prepared commercially from butanal by an aldol reaction. Working backward, we can reason that 2-ethyl-1-hexanol might come from 2-ethylhexanal by a reduction. 2-Ethylhexanal, in turn, might be prepared by catalytic reduction of 2-ethyl-2-hexenal, which is the aldol condensation product of butanal. The reactions that follow show the sequence in reverse order.
Problem 23-5
Which of the following compounds are aldol condensation products? What is the aldehyde or ketone precursor of each?
(a) 2-hydroxy-2-methylpentanal
(b) 5-ethyl-4-methyl-4-hepten-3-one
Problem 23-6
Butanol is prepared commercially by a route that begins with an aldol reaction. Show the steps that are likely to be involved.
Problem 23-7
Show how you would synthesize the following compound using an aldol reaction: