It takes practice to use curved arrows properly in reaction mechanisms, but there are a few rules and a few common patterns you should look for that will help you become more proficient:
RULE 1
Electrons move from a nucleophilic source (Nu: or Nu:−) to an electrophilic sink (E or E+). The nucleophilic source must have an electron pair available, usually either as a lone pair or in a multiple bond. For example, electrons usually flow from one of these nucleophiles:
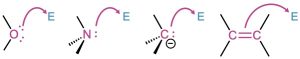
The electrophilic sink must be able to accept an electron pair, usually because it has either a positively charged atom or a positively polarized atom in a functional group. For example, electrons usually flow to one of these electrophiles.

RULE 2
The nucleophile can be either negatively charged or neutral. If the nucleophile is negatively charged, the atom that donates an electron pair becomes neutral. For example:
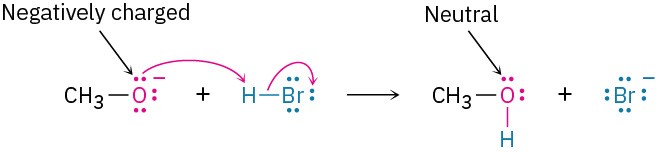
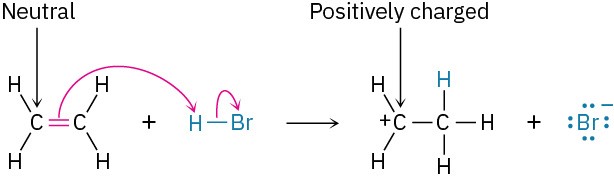
If the nucleophile is neutral, the atom that donates the electron pair acquires a positive charge. For example:
RULE 3
The electrophile can be either positively charged or neutral. If the electrophile is positively charged, the atom bearing that charge becomes neutral after accepting an electron pair. For example:
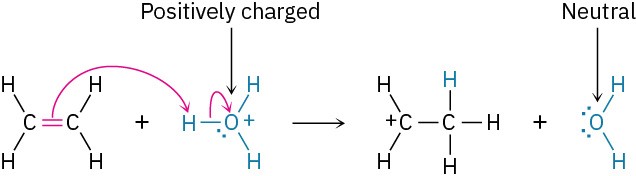
If the electrophile is neutral, the atom that ultimately accepts the electron pair acquires a negative charge. For this to happen, however, the negative charge must be stabilized by being on an electronegative atom such as oxygen, nitrogen, or a halogen. Carbon and hydrogen do not typically stabilize a negative charge. For example:
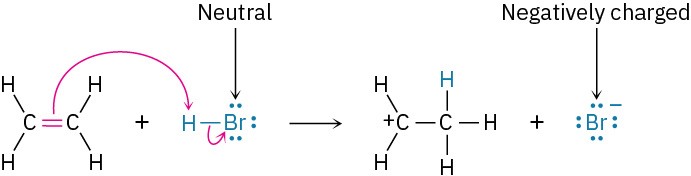
The result of Rules 2 and 3 together is that charge is conserved during the reaction. A negative charge in one of the reactants gives a negative charge in one of the products, and a positive charge in one of the reactants gives a positive charge in one of the products.
RULE 4
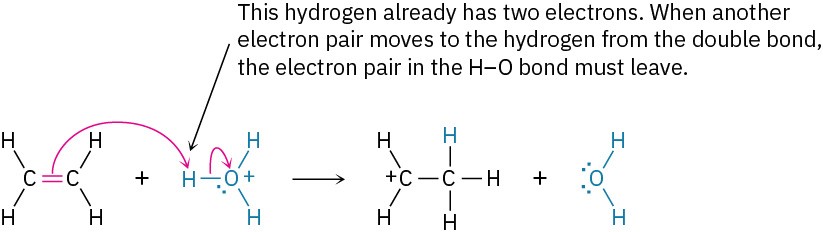
The octet rule must be followed. That is, no second-row atom can be left with ten electrons (or four for hydrogen). If an electron pair moves to an atom that already has an octet (or two electrons for hydrogen), another electron pair must simultaneously move from that atom to maintain the octet. When two electrons move from the C═C bond of ethylene to the hydrogen atom of H3O+, for instance, two electrons must leave that hydrogen. This means that the H−O bond must break and the electrons must stay with the oxygen, giving neutral water.
Worked Example 6.2 Using Curved Arrows in Reaction Mechanisms
Add curved arrows to the following polar reaction to show the flow of electrons:
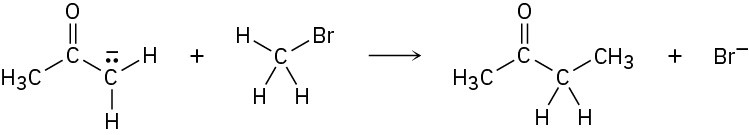
Strategy
Look at the reaction, and identify the bonding changes that have occurred. In this case, a C−Br bond has broken and a C−C bond has formed. The formation of the C−C bond involves donation of an electron pair from the nucleophilic carbon atom of the reactant on the left to the electrophilic carbon atom of CH3Br, so we draw a curved arrow originating from the lone pair on the negatively charged C atom and pointing to the C atom of CH3Br. At the same time that the C−C bond forms, the C−Br bond must break so that the octet rule is not violated. We therefore draw a second curved arrow from the C−Br bond to Br. The bromine is now a stable Br− ion.
Solution
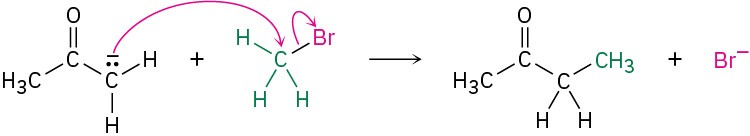
Problem 6-6
Add curved arrows to the following polar reactions to indicate the flow of electrons in each:
(a) 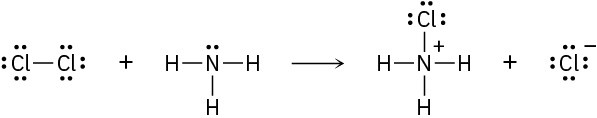
(b) 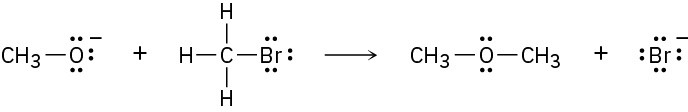
(c) 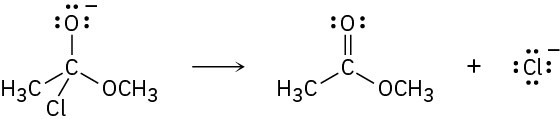
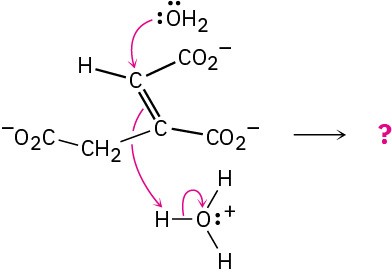
Problem 6-7
Predict the products of the following polar reaction, a step in the citric acid cycle for food metabolism, by interpreting the flow of electrons indicated by the curved arrows: