Figure 11.1 Competition occurs throughout nature. In chemistry, competition often occurs between alternative reaction pathways, such as in the substitution and elimination reactions of alkyl halides. (credit: modification of work “Bull moose fight” by Grand Teton, National Parks Service/Flickr, Public Domain)
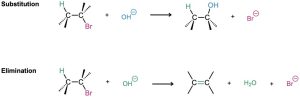
Nucleophilic substitution and base-induced elimination are two of the most widely occurring and versatile reactions in organic chemistry, both in the laboratory and in biological pathways. We’ll look at them closely in this chapter to see how they occur, what their characteristics are, and how they can be used. We’ll begin with substitution reactions.
We saw in the preceding chapter that the carbon–halogen bond in an alkyl halide is polar and that the carbon atom is electron-poor. Thus, alkyl halides are electrophiles, and much of their chemistry involves polar reactions with nucleophiles and bases. Alkyl halides do one of two things when they react with a nucleophile/base such as hydroxide ion: they either undergo substitution of the X group by the nucleophile, or they undergo elimination of HX to yield an alkene.