Figure 22.1 Tear gas is a controversial riot-control device used by some military and police forces. It is a simple chloroketone made by a carbonyl α-substitution reaction. (credit: modification of work “Tear Gas in CWB Hennessy Road View” by The Stand News/Wikimedia Commons, Public Domain)
As with nucleophilic additions and nucleophilic acyl substitutions, many laboratory schemes, pharmaceutical syntheses, and biochemical pathways make frequent use of carbonyl α-substitution reactions. Their great value comes from the fact that they constitute one of the few general methods for forming carbon–carbon bonds, thereby making it possible to build larger molecules from smaller precursors. In this chapter, we’ll see how and why these reactions occur.
We said in the Preview of Carbonyl Chemistry that much of the chemistry of carbonyl compounds can be explained by just four fundamental reaction types: nucleophilic additions, nucleophilic acyl substitutions, α-substitutions, and carbonyl condensations. Having studied the first two of these reactions in the past three chapters, let’s now look in more detail at the third major carbonyl-group process—the α-substitution reaction.
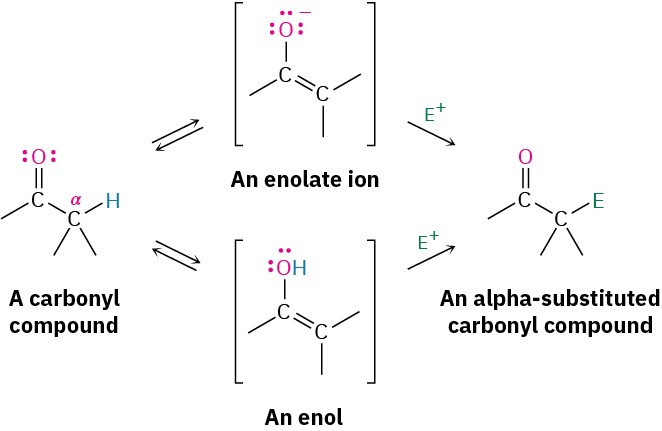
Alpha-substitution reactions occur at the position next to the carbonyl group—the α position—and involve the substitution of an α hydrogen atom by an electrophile, E, through either an enol or enolate ion intermediate. Let’s begin by learning more about these two species.