Figure 31.1 If you ride a bike, wear your helmet! Most bike helmets are made of two different polymers, a hard polycarbonate shell and an inner layer of polystyrene. (credit: modification of work “View from the top” by Gene Bisbee/Flickr, CC BY 2.0)
Our treatment of polymers has thus far been dispersed over several chapters, but it’s also important to take a comprehensive view. In the present chapter, we’ll look further at how polymers are made, and we’ll see how polymer structure correlates with physical properties. No course in organic chemistry would be complete without a look at polymers.
Polymers are a fundamental part of the modern world, used in everything from coffee cups to cars to clothing. In medicine, too, their importance is growing, with uses as diverse as cardiac pacemakers, artificial heart valves, and biodegradable sutures.
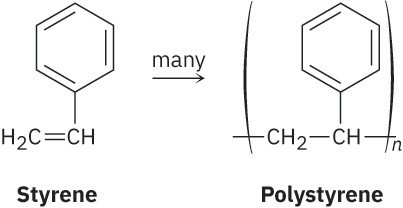
We’ve seen on several occasions in previous chapters that a polymer, whether synthetic or biological, is a large molecule built up by repetitive bonding of many smaller units, or monomers. Polyethylene, for instance, is a synthetic polymer made from ethylene (Section 8.10), nylon is a synthetic polyamide made from a diacid and a diamine (Section 21.9), and proteins are biological polyamides made from amino acids. Note that polymers are often drawn by indicating their repeating unit in parentheses. The repeating unit in polystyrene, for example, comes from the monomer styrene.