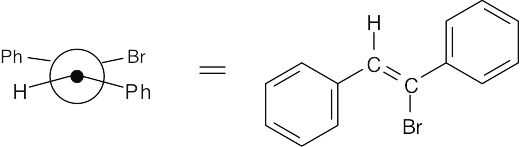
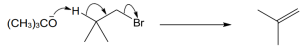
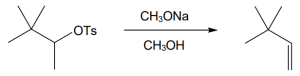
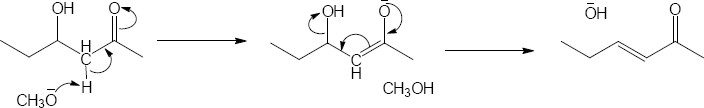
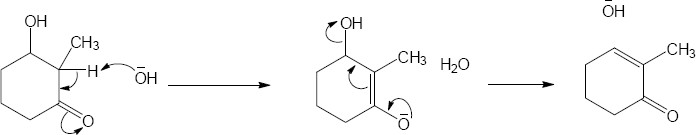
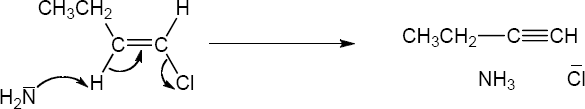
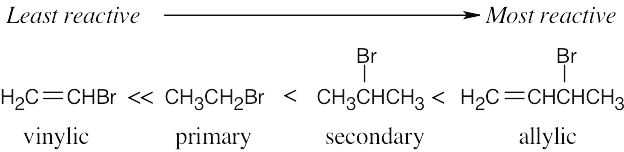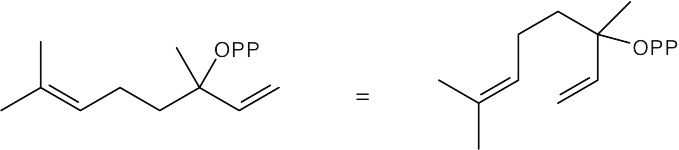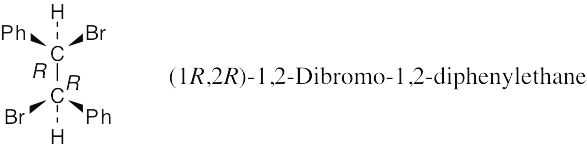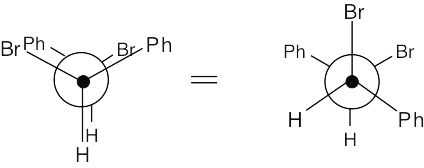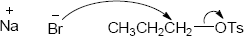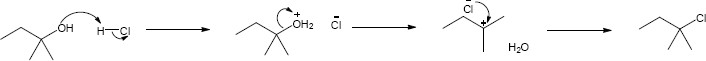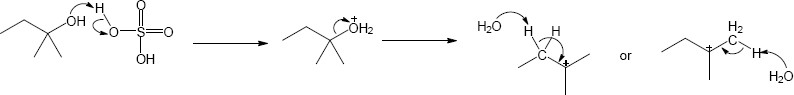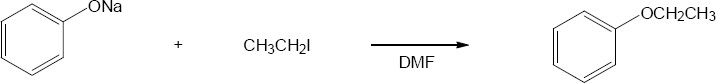11 Chapter 11 – Reactions of Alkyl Halides: Nucleophilic Substitutions and
| 11.1 |
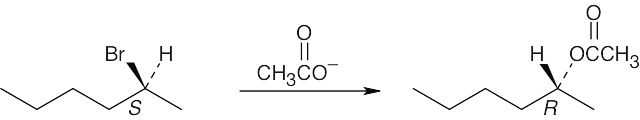
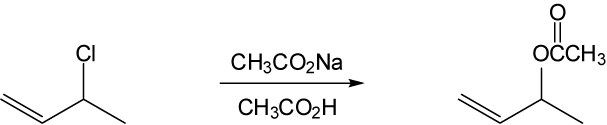
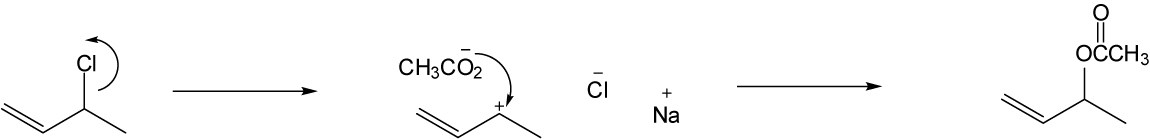
As described in Worked Example 11.1, identify the leaving group and the chirality center. Draw the product carbon skeleton, inverting the configuration at the chirality center, and replace the leaving group (bromide) with the nucleophilic reactant (acetate).
|
| 11.2 |
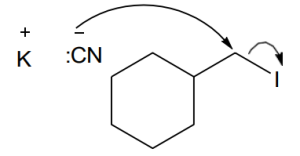
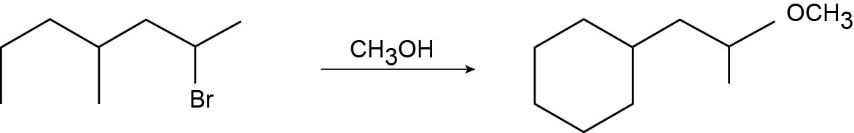
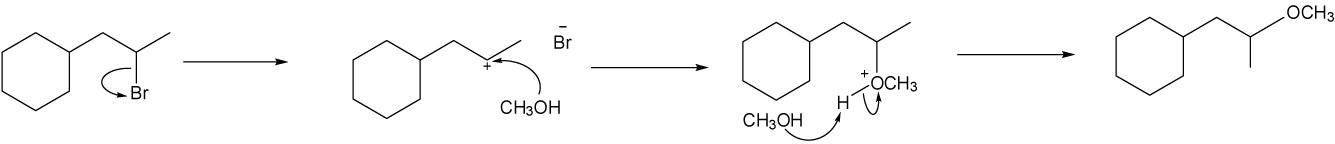
Use the suggestions in the previous problem to draw the correct product.
|
| 11.3 |
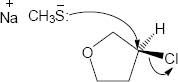
|
| 11.4 | All of the nucleophiles in this problem are relatively reactive. See Table 11.1. | |
| (a) | CH3CH2CH2CH2Br + NaI ⎯⎯→ CH3CH2CH2CH2I + NaBr | |
| (b) | CH3CH2CH2CH2Br + KOH ⎯⎯→ CH3CH2CHCH2 + KBr | |
| (c) | CH3CH2CH2CH2Br + HC≡C– Li+ ⎯⎯→ CH3CH2CH2CH2C≡CH + LiBr | |
| (d) | CH3CH2CH2CH2Br + NH3 ⎯⎯→ CH3CH2CH2CH2NH3 + Br– | |
| 11.5 | (a) | (CH3)2N– is more nucleophilic because it is more basic than (CH3)2NH and because a negatively charged nucleophile is more nucleophilic than a neutral nucleophile. |
| (b) | (CH3)3N is more nucleophilic than (CH3)3B. (CH3)3B is non-nucleophilic because it has no lone electron pair. | |
| (c) | H2S is more nucleophilic than H2O because nucleophilicity increases in going down a column of the periodic table. |
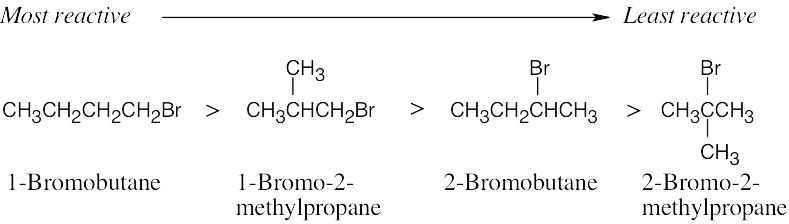
| 11.6 | In this problem, we are comparing two effects – the effect of the substrate and the effect of the leaving group. Tertiary substrates are less reactive than secondary substrates, which are less reactive than primary substrates. | ||||||||||||||
| Least reactive ⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯⎯→ Most reactive | |||||||||||||||
|
| 11.7 |
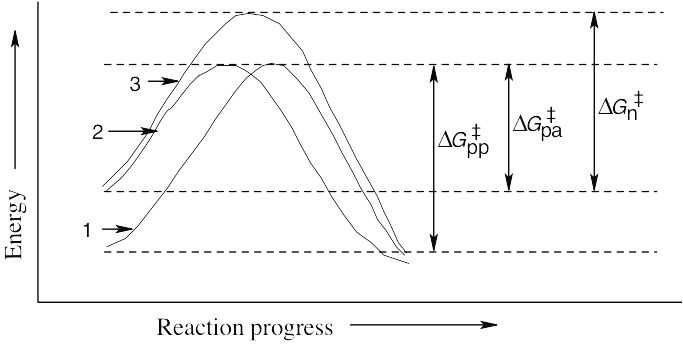
Polar protic solvents (curve 1) stabilize the charged transition state by solvation and also stabilize the nucleophile by hydrogen bonding. Polar aprotic solvents (curve 2) stabilize the charged transition state by solvation, but do not hydrogen-bond to the nucleophile. Since the energy level of the nucleophile is higher, ΔG‡ is smaller and the reaction is faster in polar aprotic solvents than in polar protic solvents. Nonpolar solvents (curve 3) stabilize neither the nucleophile nor the transition state. ΔG‡ is therefore higher in nonpolar solvents than in polar solvents, and the reaction rate is slower. Benzene, ether, and chloroform are in this category. |
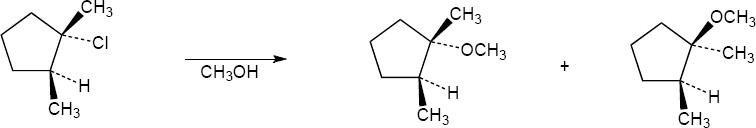
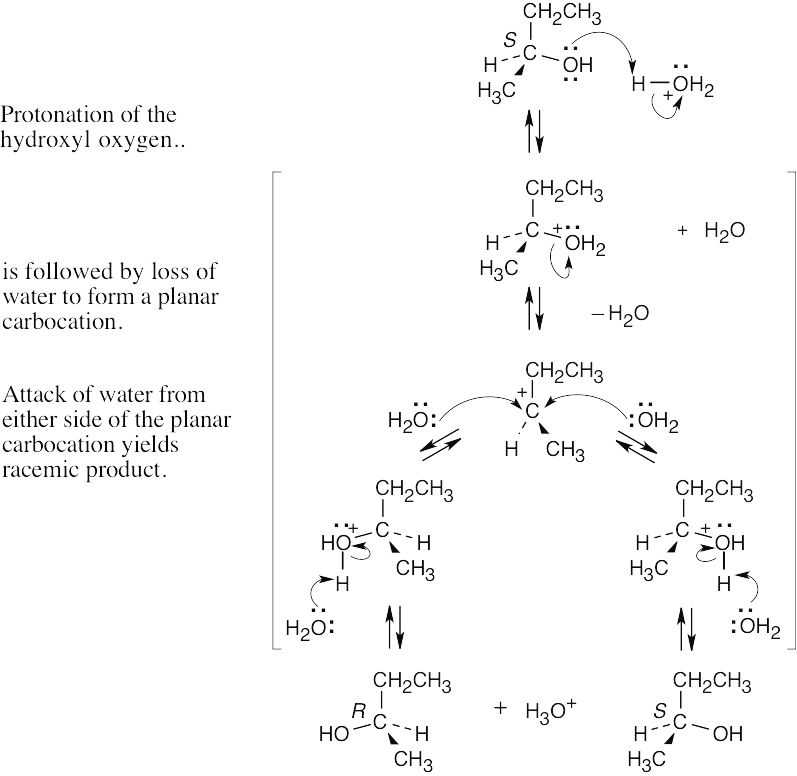
| 11.8 | 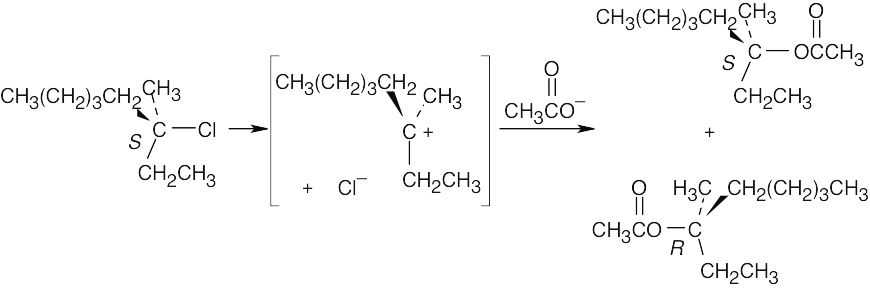
In this SN1 reaction, attack by acetate can occur on either side of the planar, achiral carbocation intermediate, resulting in a mixture of both the R and S enantiomeric acetates. The ratio of enantiomers is probably close to 50:50. |
| 11.9 | If reaction had proceeded with complete inversion, the product would have had a specific rotation of +53.6°. If complete racemization had occurred, [α]D would have been zero. The observed rotation was +5.3°. Since [latex]\frac{+5.3°}{+53.6°} = 0.099[/latex], 9.9% of the original tosylate was inverted. The remaining 90.1% of the product must have been racemized. |
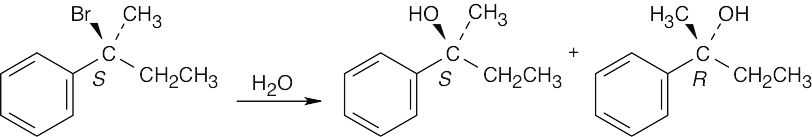
| 11.10 | The S substrate reacts with water to form a mixture of R and S alcohols. The ratio of enantiomers is close to 50:50.
|
| 11.11 | SN1 reactivity is related to carbocation stability. Thus, substrates that form the most stable carbocations are the most reactive in SN1 reactions.
|
| 11.12 | 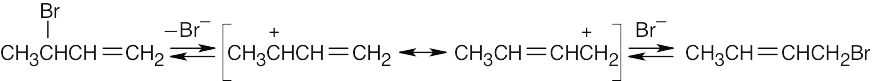
The two bromobutenes form the same allylic carbocation in the rate-limiting step. |
| 11.13 | Both substrates have allylic groups and might react either by an SN1 or an SN2 route. The reaction mechanism is determined by the leaving group, the solvent, or the nucleophile. | |
| (a) | This reaction probably occurs by an SN1 mechanism. HCl converts the poor –OH leaving group into an excellent –OH2+ leaving group, and the polar solvent stabilizes the carbocation intermediate. | |
| (b) | This reaction takes place with a negatively charged nucleophile in a polar, aprotic solvent. It is very likely that the reaction occurs by an SN2 mechanism. | |
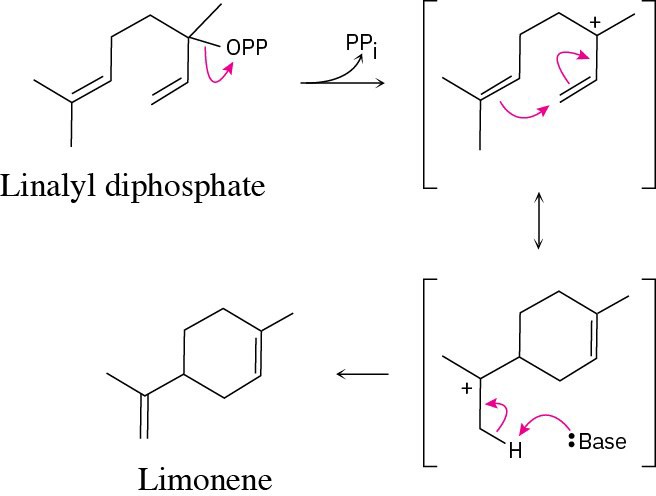
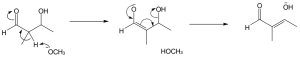
| 11.14 | Redraw linalyl diphosphate so that it has the same orientation as limonene.
After dissociation of PPi, the cation cyclizes by attack of the double bond π electrons. Removal of –H by base yields limonene.
|
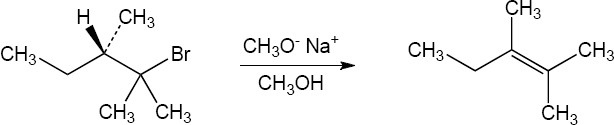
| 11.15 | Form the double bond by removing HX from the alkyl halide reactant in as many ways as possible. The major elimination product in each case has the most substituted double bond (Zaitsev’s rule). | |
| (a) | 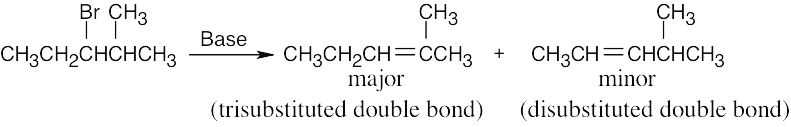 |
|
| (b) | 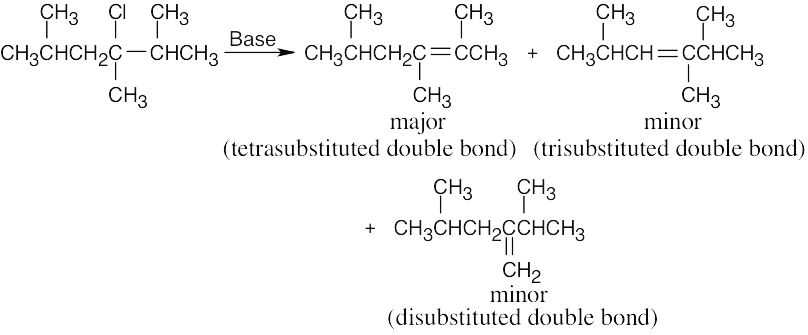 |
|
| (c) | 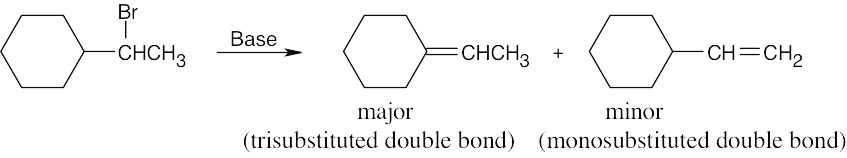 |
|
| 11.16 | For maximum yield, the alkyl halide reactant should not give a mixture of products on elimination. | |
| (a) | 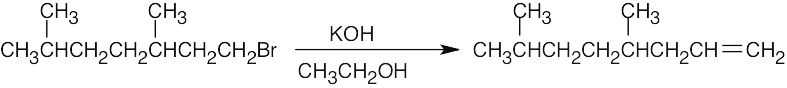
The 2-bromo isomer yields a mixture of alkene products. |
|
| (b) | 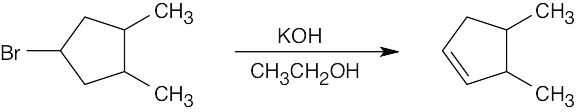 |
|
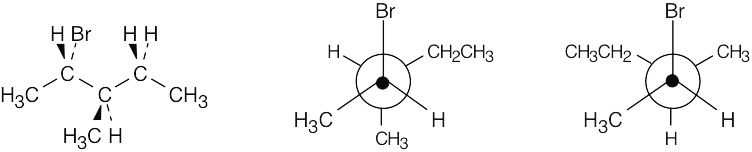
| 11.17 | Draw the reactant with correct stereochemistry.
Convert this drawing into a Newman projection, and draw the conformation having anti periplanar geometry (staggered) for –H and –Br.
The alkene resulting from E2 elimination is (Z)-1-bromo-1,2-diphenylethylene.
|
| 11.18 |
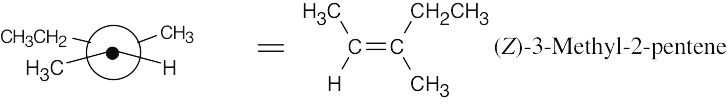
As in the previous problem, draw the structure, convert it to a Newman projection, and rotate the groups so that the –H and –Br to be eliminated have an anti-periplanar (staggered) relationship.
The major product is (Z)-3-methyl-2-pentene. A small amount of 3-methyl-1-pentene is also formed.
|
| 11.19 | 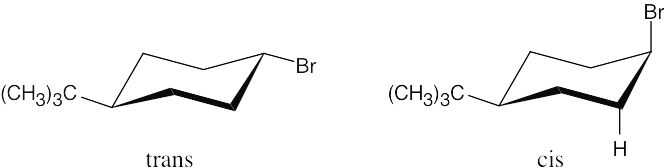
The more stable conformations of each of the two isomers are pictured above; the larger tert–butyl group is always equatorial in the more stable conformation. The cis isomer reacts faster under E2 conditions because –Br and –H are in the anti-periplanar arrangement that favors E2 elimination. |
| 11.20 | (a) | 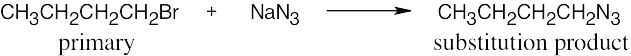
The reaction occurs by an SN2 mechanism because the substrate is primary, the nucleophile is nonbasic, and the product is a substitution product. |
| (b) | 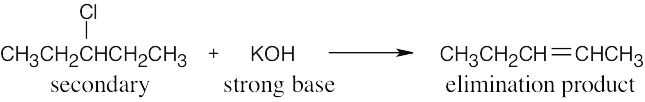
This is an E2 reaction since a secondary halide reacts with a strong base to yield an elimination product. |
|
| (c) | 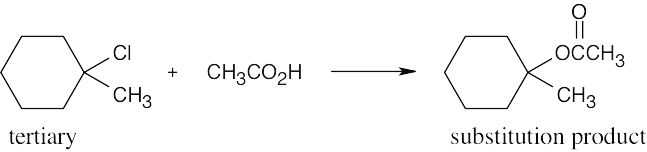
This is an SN1 reaction. Tertiary substrates form substitution products only by the SN1 route. |
|
| (d) | 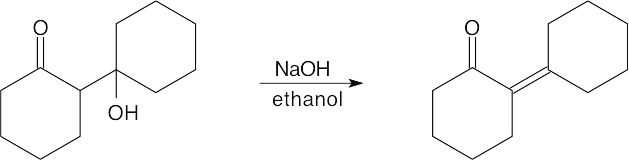
This is an E1cB reaction because the leaving group is two carbons away from a carbonyl group. |
Additional Problems
Visualizing Chemistry
| 11.21 | (a) | (i) | CH3CH2Cl + Na+ –SCH3 ⎯⎯→ CH3CH2SCH3 + NaCl |
| (ii) | CH3CH2Cl + Na+ –OH ⎯⎯→ CH3CH2OH + NaCl | ||
| Both reactions yield SN2 substitution products because the substrate is primary and both nucleophiles are strong. | |||
| (b) | 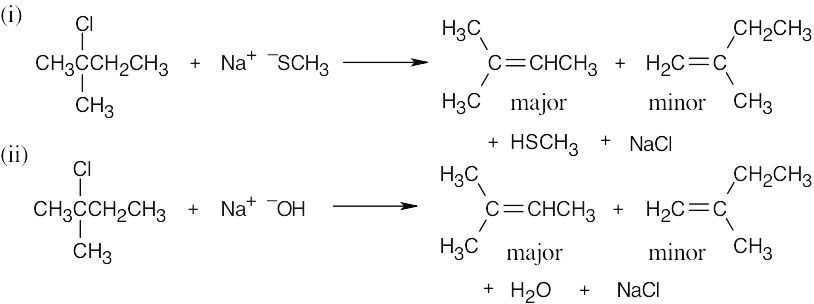
The substrate is tertiary, and the nucleophiles are basic. Two elimination products are expected; the major product has the more substituted double bond, in accordance with Zaitsev’s rule. |
||
| (c) | 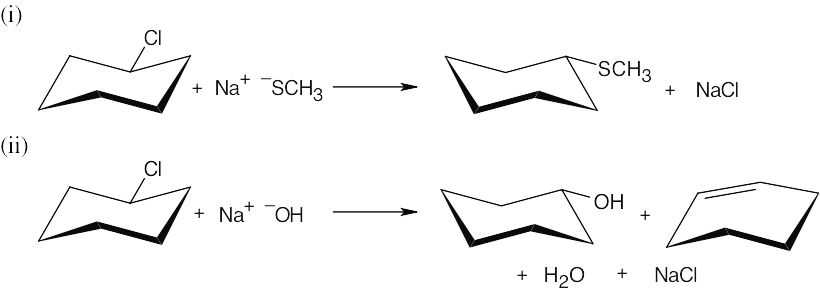
In (i), the secondary substrate reacts with the good, but weakly basic, nucleophile to yield substitution product. In (ii), NaOH is a poorer nucleophile but a stronger base, and both substitution and elimination product are formed. |
||
| 11.22 | 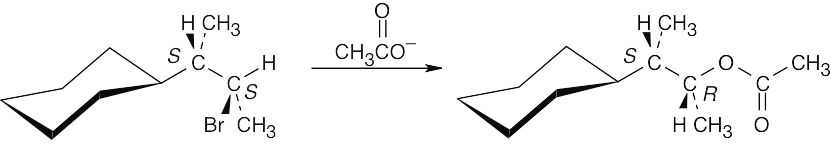
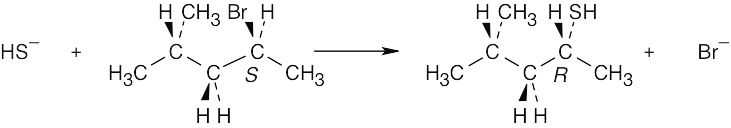
Reaction of the secondary bromide with the weakly basic acetate nucleophile occurs by an SN2 route, with inversion of configuration, to produce the R acetate. |
| 11.23 | 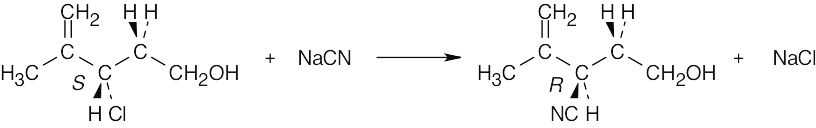
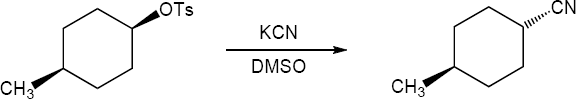
The S substrate has a secondary allylic chloride group and a primary hydroxyl group. SN2 reaction occurs at the secondary carbon to give the R cyano product because hydroxide is a poor leaving group. |
| 11.24 | 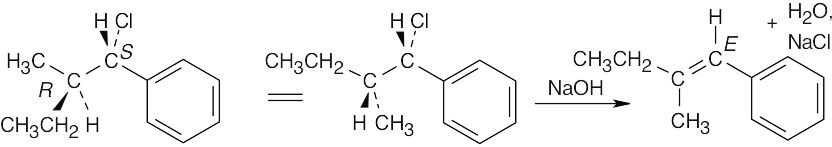
Rotate the left side of the molecule so that the groups to be eliminated have an anti-periplanar relationship. The double bond in the product has the E configuration. |
Mechanism Problems
| 11.25 | The mechanism for each problem is SN2. | |
| (a) | The electrophile is a 1° alkyl halide reacting with a good nucleophile.
Mechanism:
|
|
| (b) | The electrophile is a 1° alkyl halide reacting with a good nucleophile.
Mechanism:
|
|
| (c) | The electrophile is a 1° alkyl halide reacting with a good nucleophile.
Mechanism:
|
|
| (d) | The electrophile is a 2° alkyl halide reacting with a good nucleophile.
Mechanism:
|
|
| 11.26 | Each mechanism goes through an SN1 mechanism. In each case there is opportunity to form a carbocation prior to substitution. | |
| (a) | Formation of a tertiary carbocation
Mechanism:
|
|
| (b) | Formation of a resonance stabilized carbocation
Mechanism:
|
|
| (c) | Use of a poor nucleophilic polar, protic solvent
Mechanism:
|
|
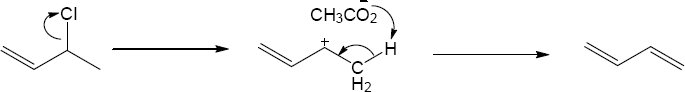
| 11.27 | In each case the mechanism is E1 because a carbocation can be formed before the proton is removed. | |
| (a) | 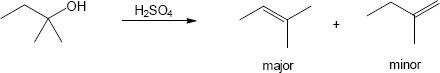
Mechanism:
|
|
| (b) | 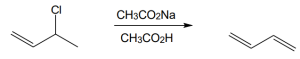
Mechanism:
|
|
| (c) | 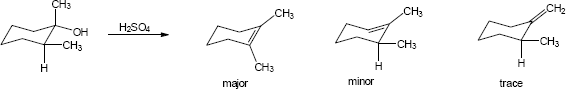
Mechanism:
|
|
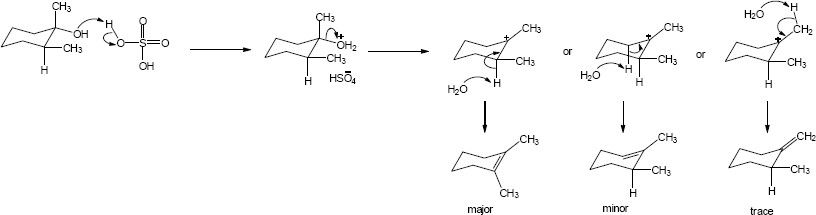
| 11.28 | Each mechanism is an E2 elimination. | |
| (a) |
E2 involving a strong base and a secondary alkyl halide. The E2 mechanism requires the leaving group and H to be removed to be anti-periplanar to one another. In the chair conformation that means that the leaving group and the proton must be axial. Only the less substituted product is formed because only the bold proton is anti-periplanar to the Cl.
Mechanism:
|
|
| (b) | E2 involving a strong, bulky base and a primary alkyl halide
Mechanism:
|
|
| (c) | E2 involving a strong base and a secondary alkyl halide\
Mechanism:
|
|
| 11.29 | Each mechanism is E1cB. In each case there is an OH β to (two carbons from) the carbonyl in the presence of a strong base. | |
| (a) | 
Mechanism:
|
|
| (b) | 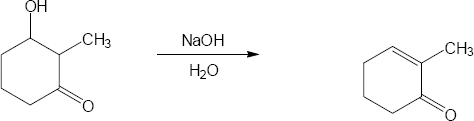
Mechanism:
|
|
| (c) | 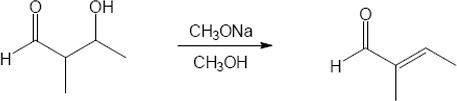
Mechanism:
|
|
| 11.30 | (a) |
In this reaction a β-hydroxyketone reacts with a strong base. These are the conditions necessary for an E1cB mechanism.
|
| (b) |
In this reaction a primary alkyl halide reacts with a good nucleophile. These are the conditions necessary for an SN2 mechanism.
|
|
| (c) |
In this reaction a tertiary alkyl halide reacts with a poor nucleophile which is a polar protic solvent. These are the conditions that favor for an SN1 mechanism.
|
|
| (d) |
In this reaction a tertiary alkyl halide reacts with a strong base. These are the conditions that favor for an E2 mechanism. Even though a tertiary alkyl halide and a polar protic solvent are present, the strong base results in elimination much faster than the formation of a carbocation.
|
| 11.31 | 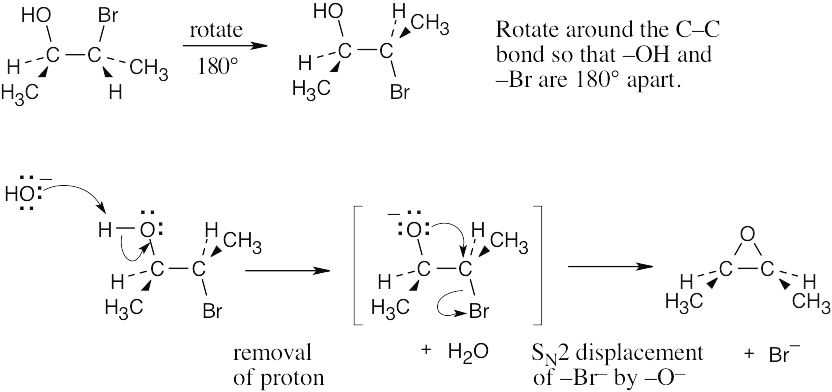
This reaction is an intramolecular SN2 displacement. |
| 11.32 | The first step in an SN1 displacement is dissociation of the substrate to form a planar, sp2- hybridized carbocation and a leaving group. The carbocation that would form from dissociation of this alkyl halide cannot become planar because of the rigid structure of the ring skeleton. Because it is not possible to form the necessary carbocation, an SN1 reaction cannot occur. In addition, approach by a nucleophile from the back side of the alkyl halide is blocked by the rigid ring system, and SN2 displacement cannot take place either. |
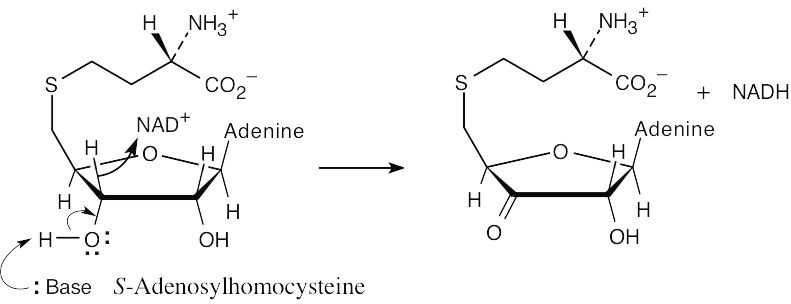
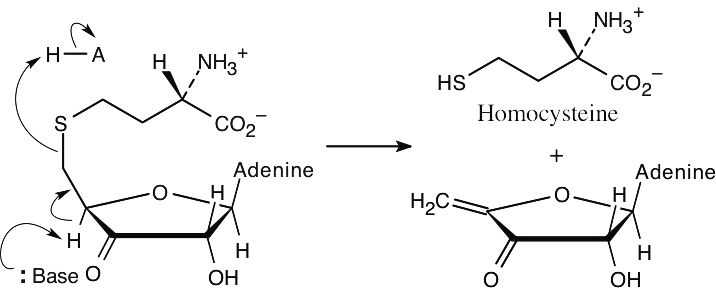
| 11.33 | Step 1: | NAD+ oxidizes an alcohol to a ketone.
|
| Step 2: | Base brings about an E1cB elimination reaction that has homocysteine as the leaving group.
|
| 11.34 | 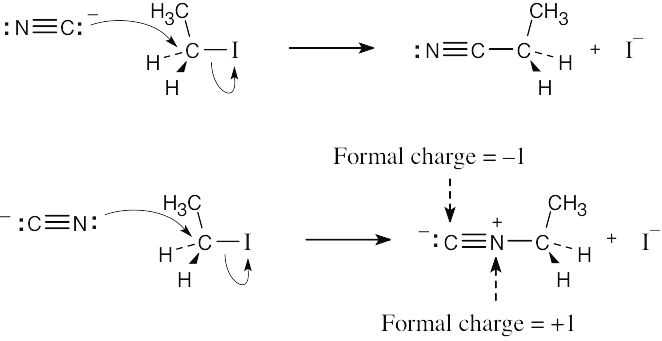
SN2 attack by the lone pair electrons associated with carbon gives the nitrile product. Attack by the lone pair electrons associated with nitrogen yields the isonitrile product. |
| 11.35 | 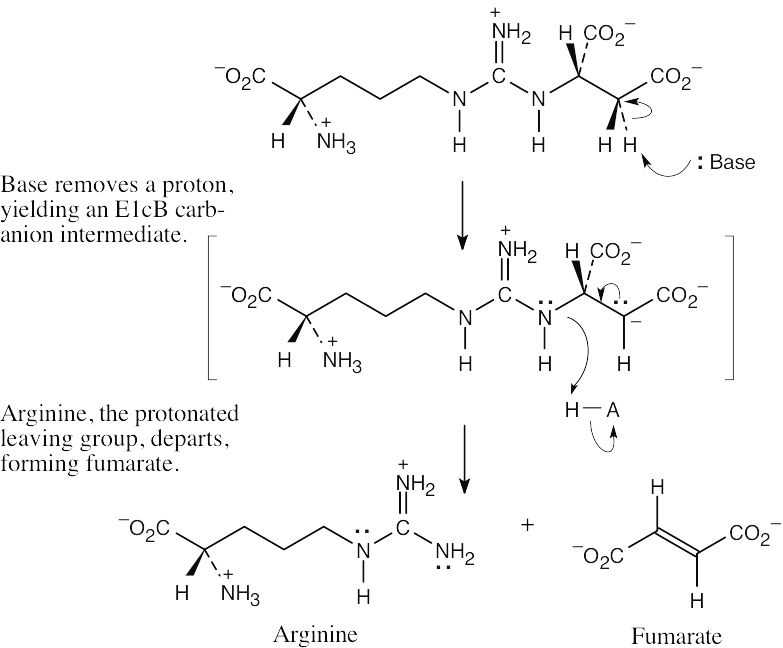 |
| 11.36 |
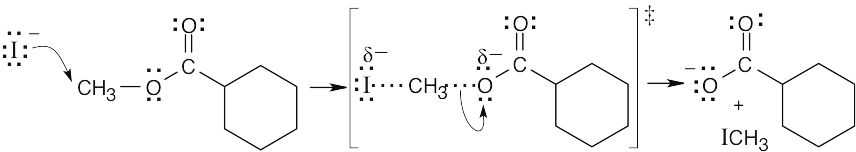
The two pieces of evidence indicate that the reaction proceeds by an SN2 mechanism: SN2 reactions proceed much faster in polar aprotic solvents such as DMF, and methyl esters react faster than ethyl esters. This reaction is an SN2 displacement on a methyl ester by iodide ion.
Other experiments can provide additional evidence for an SN2 mechanism. We can determine if the reaction is second-order by varying the concentration of LiI. We can also vary the type of nucleophile to distinguish an SN2 mechanism from an SN1 mechanism, which does not depend on the identity of the nucleophile. |
| 11.37 | 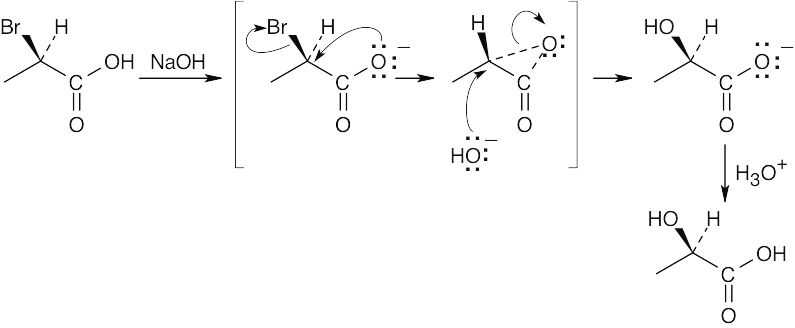
Two inversions of configuration equal a net retention of configuration. |
| 11.38 | 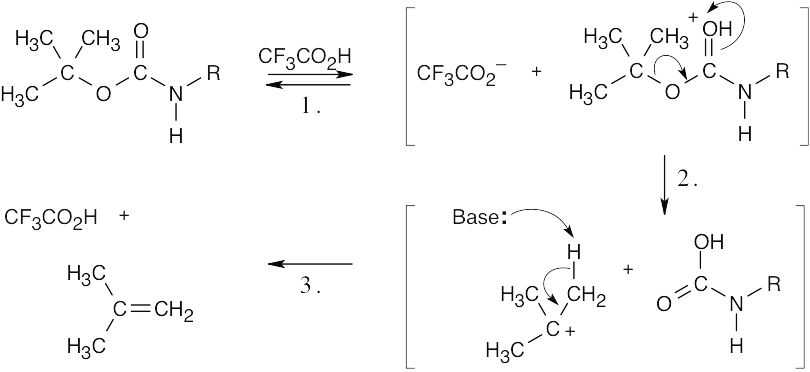 |
|
| Step 1: | Protonation. | |
| Step 2: | Elimination of leaving group. | |
| Step 3: | Removal of proton by base. | |
| This reaction proceeds by an E1 mechanism. | ||
| 11.39 |
|
| 11.40 |
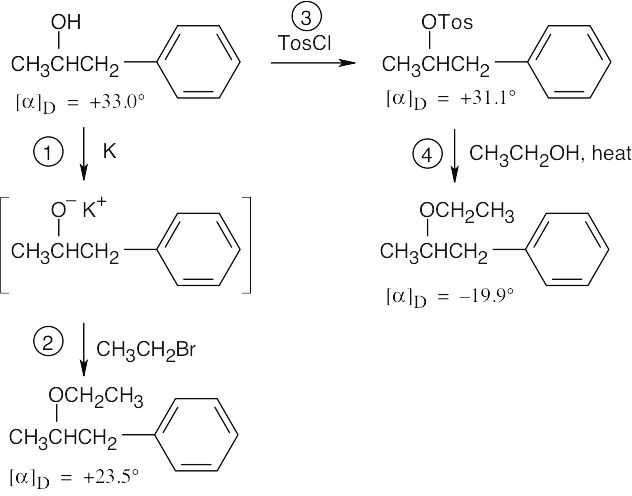
An alcohol is converted to an ether by two different routes in this series of reactions. The two resulting ethers have identical structural formulas but differ in the sign of specific rotation. Therefore, at some step or steps in these reaction sequences, inversion of configuration at the chiral carbon must have occurred. Let’s study each step of the series to find where inversion is occurring.
In step 1, the alcohol reacts with potassium metal to produce a potassium alkoxide. Since the bond between carbon and oxygen has not been broken, no inversion occurs in this step. The potassium alkoxide acts as a nucleophile in the SN2 displacement on CH3CH2Br in step 2. It is the C–Br bond of bromoethane, however, not the C–O bond of the alkoxide that is broken. No inversion at the carbon chirality center occurs in step 2. The starting alcohol reacts with tosyl chloride in step 3. Again, because the O–H bond, rather than the C–O bond, of the alcohol is broken, no inversion occurs at this step. Inversion must therefore occur at step 4 when the –OTos group is displaced by CH3CH2OH. The C–O bond of the tosylate (–OTos) is broken, and a new C–O bond is formed. Notice the specific rotations of the two enantiomeric products. The product of steps 1 and 2 should be enantiomerically pure because neither reaction has affected the C–O bond. Reaction 4 proceeds with some racemization at the chirality center to give a smaller absolute value of [α]D. |
| 11.41 | (a) | CH3I reacts faster than CH3Br because I– is a better leaving group than Br–. |
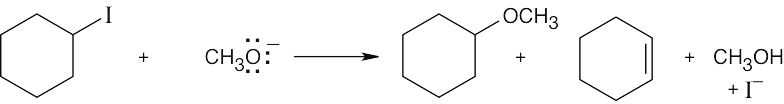
| (b) | CH3CH2I reacts faster with OH– in dimethylsulfoxide (DMSO) than in ethanol. Ethanol, a protic solvent, hydrogen-bonds with hydroxide ion and decreases its reactivity. | |
| (c) | Under the SN2 conditions of this reaction, CH3Cl reacts faster than (CH3)3CCl. Approach of the nucleophile to the bulky (CH3)3CCl molecule is hindered. | |
| (d) | H2C=CHCH2Br reacts faster because vinylic halides such as H2C=CHBr are unreactive to substitution reactions. |
| 11.42 | To predict nucleophilicity, remember these guidelines: | |||||||||||||||||||||||||||||
| (1) | In comparing nucleophiles that have the same attacking atom, nucleophilicity parallels basicity. In other words, a more basic nucleophile is a more effective nucleophile. | |||||||||||||||||||||||||||||
| (2) | Nucleophilicity increases in going down a column of the periodic table. | |||||||||||||||||||||||||||||
| (3) | A negatively charged nucleophile is usually more reactive than a neutral nucleophile. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| 11.43 | 
This is an SN2 reaction, whose rate depends on the concentration of both alkyl halide and nucleophile. Rate = k × [RX] × [Nu: –]. |
|
| (a) | Halving the concentration of cyanide ion and doubling the concentration of alkyl halide does not change the reaction rate. The two effects cancel. | |
| (b) | Tripling the concentrations of both cyanide ion and alkyl halide causes a ninefold increase in reaction rate. | |
| 11.44 | 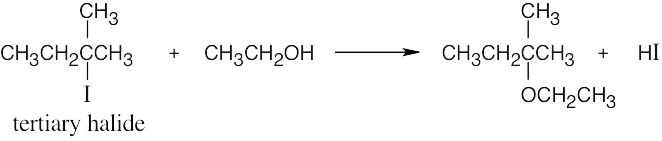
This is an SN1 reaction, whose rate depends only on the concentration of 2-iodo-2- methylbutane. Rate = k × [RX]. |
|
| (a) | Tripling the concentration of alkyl halide triples the rate of reaction. | |
| (b) | Halving the concentration of ethanol by dilution with diethyl ether reduces the polarity of the solvent and decreases the rate. | |
| 11.45 | (a) |
CH3Br + Na+ –C≡CCH(CH3)2 ⎯⎯→ CH3C≡CCH(CH3)2 + NaBr Not CH3C≡C– Na+ + BrCH(CH3)2. The strong base CH3C≡C– brings about elimination, producing CH3C≡CH and H2C=CHCH3. |
| (b) | 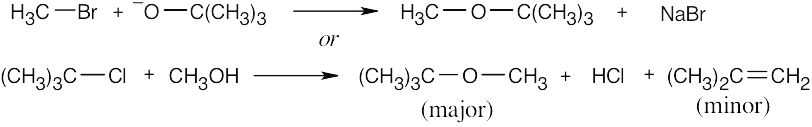 |
|
| (c) | CH3CH2CH2CH2Br + NaCN ⎯⎯→ CH3CH2CH2CH2CN + NaBr | |
| (d) | CH3CH2CH2Br + excess NH3 ⎯⎯→ CH3CH2CH2NH2 + NH4+Br– (major) |
| 11.46 | (a) | The difference in this pair of reactions is in the leaving group. Since –OTos is a better leaving group than –Cl (see Section 11.5), SN2 displacement by iodide on CH3–OTos proceeds faster. |
| (b) | The substrates in these two reactions are different. Bromoethane is a primary bromoalkane, and bromocyclohexane is a secondary bromoalkane. Since SN2 reactions proceed faster at primary than at secondary carbon atoms, SN2 displacement on bromoethane is a faster reaction. | |
| (c) | Ethoxide ion and cyanide ion are different nucleophiles. Since CN– is more reactive than CH3CH2O– in SN2 reactions, SN2 displacement on 2-bromopropane by CN– proceeds at a faster rate. | |
| (d) | The solvent in each reaction is different. The SN2 reaction on bromoethane in polar, aprotic acetonitrile proceeds faster than the reaction in nonpolar benzene. |
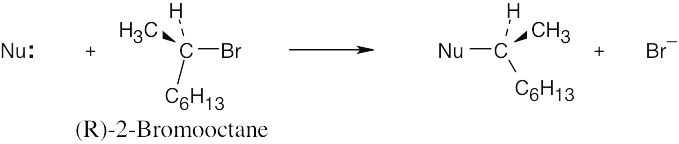
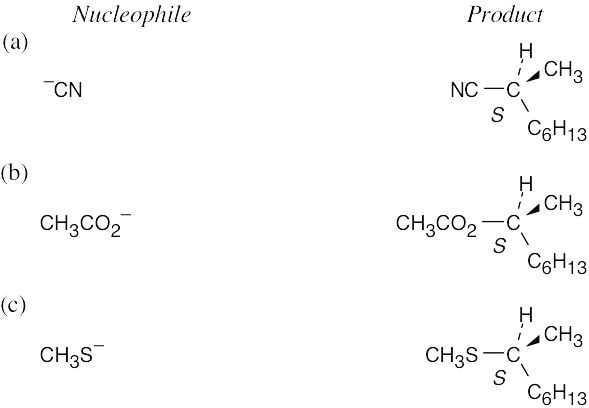
| 11.47 | (R)-2-Bromooctane is a secondary bromoalkane, which undergoes SN2 substitution. Since SN2 reactions proceed with inversion of configuration, the configuration at the carbon chirality center is inverted. (This does not necessarily mean that all R isomers become S isomers after an SN2 reaction. The R,S designation refers to the priorities of groups, which may change when the nucleophile is varied.)
|
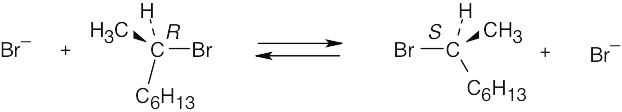
| 11.48 | After 50% of the starting material has reacted, the reaction mixture consists of 50% (R)-2-bromooctane and 50% (S)-2-bromooctane. At this point, the R starting material is completely racemized.
|
Elimination Reactions
| 11.49 | (a) | 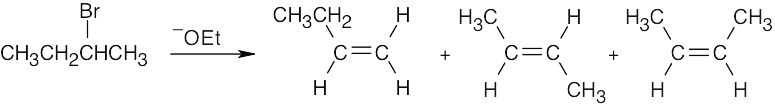 |
| (b) | H2C=CHBr, like other vinylic organohalides, does not undergo nucleophilic substitutions. | |
| (c) | 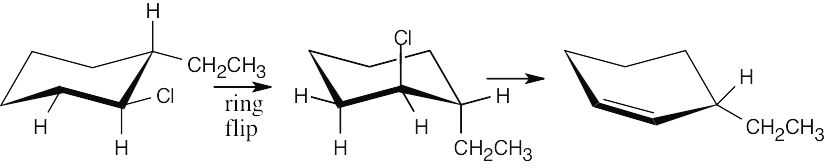
This alkyl halide gives the less substituted cycloalkene (non-Zaitsev product). Elimination to form the Zaitsev product does not occur because the –Cl and –H involved cannot assume the anti-periplanar geometry preferred for E2 elimination. |
|
| (d) |  |
| 11.50 | Because 1-bromopropane is a primary haloalkane, the reaction proceeds by either an SN2 or E2 mechanism, depending on the basicity and the amount of steric hindrance in the nucleophile. | |
| (a) |
CH3CH2CH2Br + NaNH2 ⎯⎯→ CH3CH2CH2NH2 + NaBr Propene (elimination product) is also formed because NaNH2 is a strong base. |
|
| (b) | CH3CH2CH2Br + K+ –OC(CH3)3 ⎯⎯→ CH3CH=CH2 + HOC(CH3)3 + KBr + CH3CH2CH2OC(CH3)3
K+ –OC(CH3)3 is a strong, bulky base that brings about elimination as well as some substitution. |
|
| (c) | CH3CH2CH2Br + NaI ⎯⎯→ CH3CH2CH2I + NaBr | |
| (d) | CH3CH2CH2Br + NaCN ⎯⎯→ CH3CH2CH2CN + NaBr | |
| (e) | CH3CH2CH2Br + Na+ –C≡CH ⎯⎯→ CH3CH2CH2C≡CH + NaBr | |
| (f) |  |
|
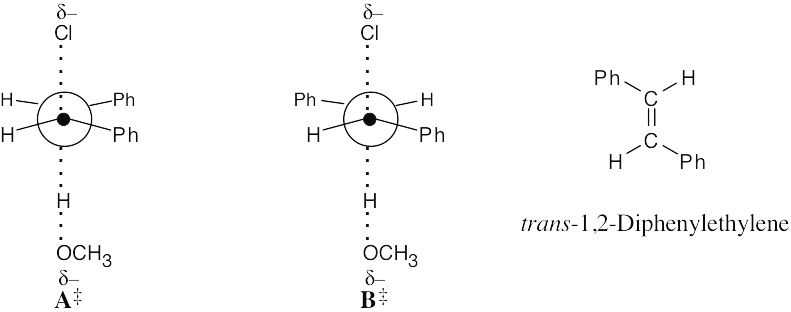
| 11.51 | 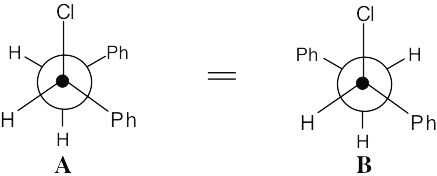
Both Newman projections place –H and –Cl in the correct anti periplanar geometry for E2 elimination.
Either transition state A‡ or B‡ can form when 1-chloro-1,2-diphenylethane undergoes E2 elimination. Crowding of the two large phenyl groups in A‡ makes this transition state (and the product resulting from it) of higher energy than transition state B‡. Formation of the product from B‡ is therefore favored, and trans-1,2-diphenylethylene is the major product. |
| 11.52 | 
The alkene shown above has the most highly substituted double bond, and, according to Zaitsev’s rule, is the major product. The following minor products may also form.
|
| 11.53 | 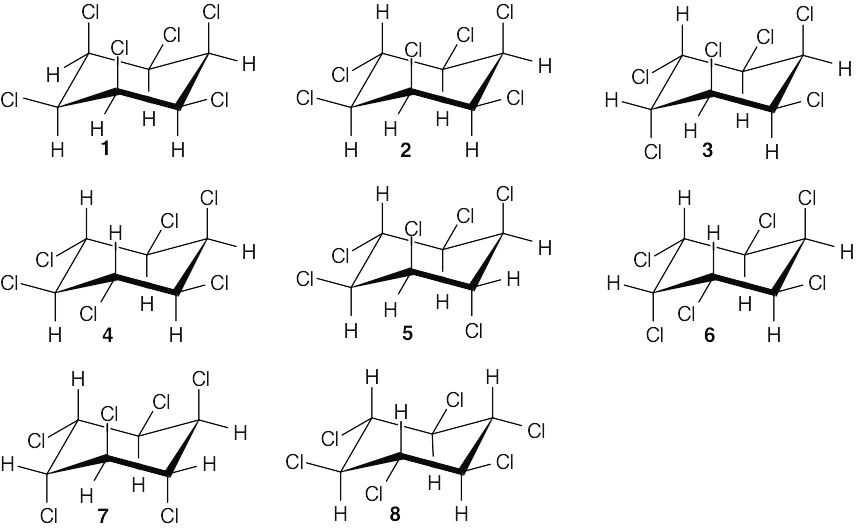
Diastereomer 8 reacts much more slowly than other isomers in an E2 reaction because no pair of hydrogen and chlorine atoms can adopt the anti-periplanar orientation preferred for E2 elimination. |
General Problems
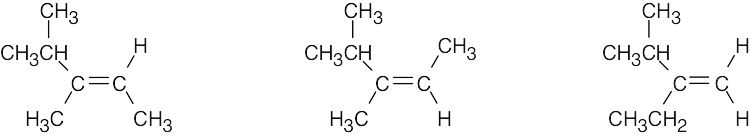
| 11.54 | (a) | Substitution does not take place with secondary alkyl halides when a strong, bulky base is used. Elimination occurs instead and produces H2C=CHCH2CH3 and CH3CH=CHCH3. |
| (b) | Fluoroalkanes do not undergo SN2 reactions because F– is a poor leaving group. | |
| (c) | SOCl2 in pyridine converts primary and secondary alcohols to chlorides by an SN2 mechanism. 1-Methyl-1-cyclohexanol is a tertiary alcohol and does not undergo SN2 substitution. Instead, E2 elimination occurs to give 1-methylcyclohexene. |
| 11.55 | (a) |  |
| (b) |  |
|
| (c) | 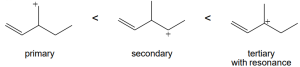 |
| 11.56 | SN1 reactivity: | |
 |
||
| (a) | 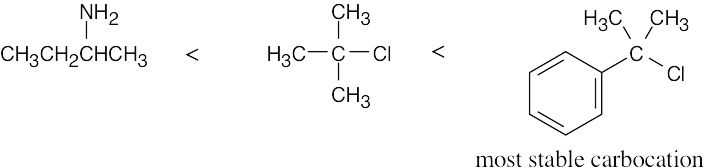 |
|
| (b) | 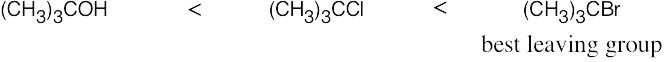 |
|
| (c) | 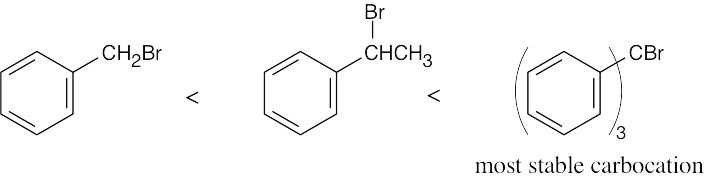 |
|
| 11.57 | SN2 reactivity: | |
 |
||
| (a) | 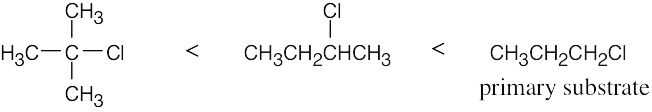 |
|
| (b) | 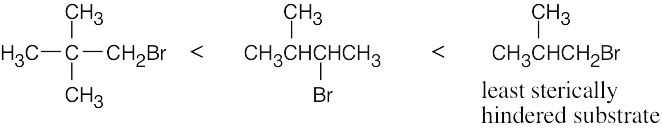 |
|
| (c) | 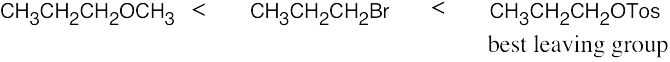 |
|
| 11.58 | (a) |
The product of this SN2 reaction (2° alkyl halide, good nucleophile) is achiral (no chiral centers). Therefore, it will not rotate plane polarized light.
|
| (b) |
This is an E2 reaction (3° alkyl halide, strong base) and the product achiral (no chiral centers). Therefore, it will not rotate plane polarized light.
|
|
| (c) |
This is an SN1 reaction (3° alkyl halide, polar protic solvent/nucleophile) and results in a pair of diastereomers. Therefore, the product mixture will rotate plane polarized light.
|
| 11.59 |
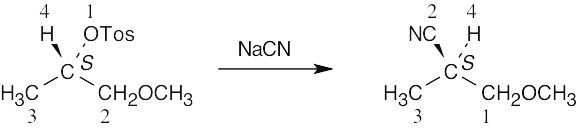
According to Cahn–Ingold–Prelog rules (Section 5.5), the nucleophile (–CN) has a lower priority than the leaving group (–OTos). Thus, even though the reaction proceeds with inversion of configuration, the priorities of the substituents also change, and the configuration remains S.
|
| 11.60 | 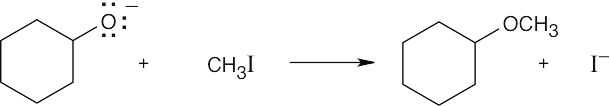
This is an excellent method of ether preparation because iodomethane is very reactive in SN2 displacements.
Reaction of a secondary haloalkane with a basic nucleophile yields both substitution and elimination products. This is a less satisfactory method of ether preparation. |
| 11.61 | 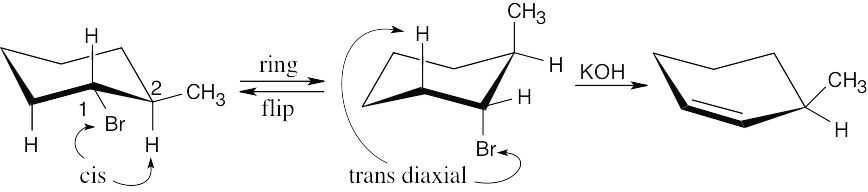
E2 reactions require that the two atoms to be eliminated have a periplanar relationship. Since it is impossible for bromine at C1 and the hydrogen at C2 to be periplanar, elimination occurs in the non-Zaitsev direction to yield 3-methylcyclohexene. |
| 11.62 | 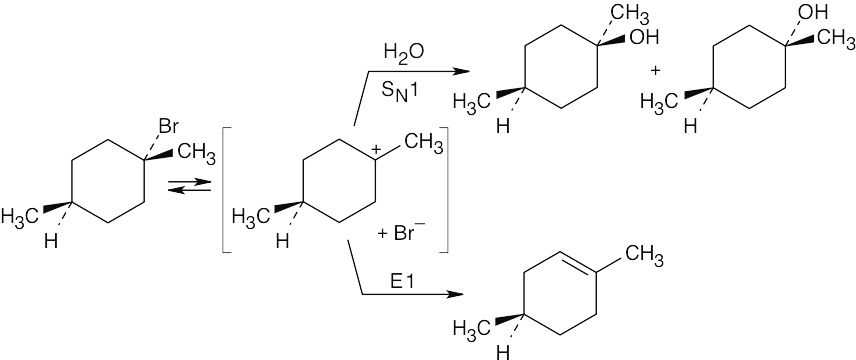
This tertiary bromoalkane reacts by SN1 and E1 routes to yield alcohol and alkene products. The alcohol products are diastereomers. |
| 11.63 | 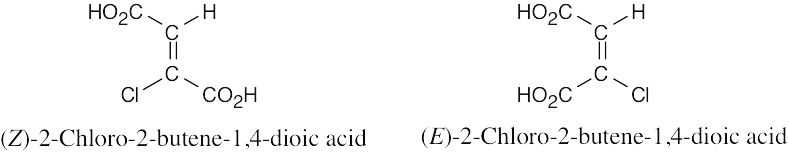
Hydrogen and chlorine are anti to each other in the Z isomer and are syn in the E isomer. Since the Z isomer reacts fifty times faster than the E isomer, elimination must proceed more favorably when the substituents to be eliminated are anti to one another. This is the same stereochemical result that occurs in E2 eliminations of alkyl halides. |
| 11.64 | (a) | 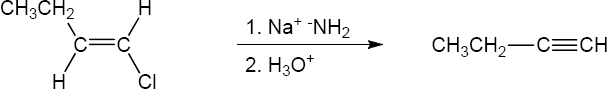
Mechanism:
|
| (b) | 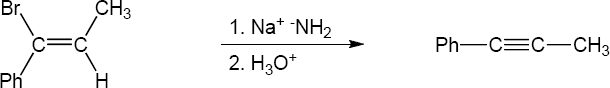
Mechanism:
|
|
| (c) | 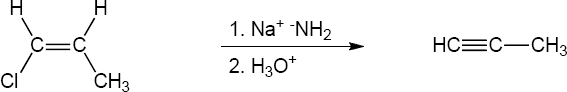
Mechanism:
|
| 11.65 | Since 2-butanol is a secondary alcohol, substitution can occur by either an SN1 or SN2 route, depending on reaction conditions. Two factors favor an SN1 mechanism in this case. (1) The reaction is run under acidic conditions in a polar, protic solvent (water). (2) Dilute acid converts a poor leaving group (–OH) into a good leaving group (OH2), which dissociates easily.
|
| 11.66 | The chiral tertiary alcohol (R)-3-methyl-3-hexanol reacts with HBr by an SN1 pathway. HBr protonates the hydroxyl group, which dissociates to yield a planar, achiral carbocation. Reaction with the nucleophilic bromide anion can occur from either side of the carbocation to produce (±)3-bromo-3-methylhexane. |
| 11.67 | Since carbon-deuterium bonds are slightly stronger than carbon-hydrogen bonds, more energy is required to break a C–D bond than to break a C–H bond. In a reaction where either a carbon-deuterium or a carbon–hydrogen bond can be broken in the rate- limiting step, a higher percentage of C–H bond-breaking occurs because the energy of activation for C–H breakage is lower.
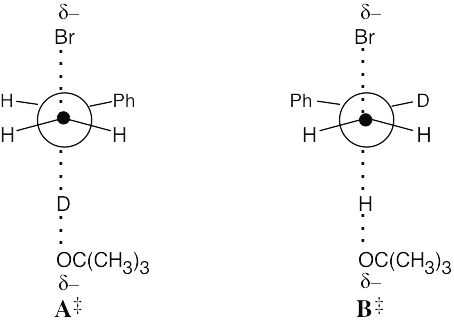
Transition state A‡ is of higher energy than transition state B‡ because more energy is required to break the C–D bond. The product that results from transition state B‡ is thus formed in greater abundance. |
| 11.68 | 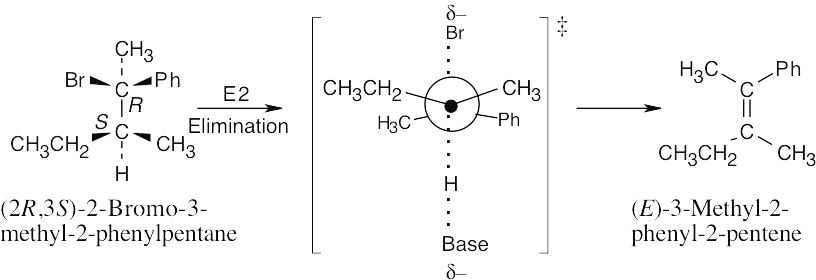
The (2S,3R) isomer also yields E product. |
| 11.69 | One of the steric requirements of E2 elimination is the need for periplanar geometry, which optimizes orbital overlap in the transition state leading to alkene product. Two types of periplanar arrangements of substituents are possible — syn and anti.
A model of the deuterated bromo compound shows that the deuterium, bromine, and the two carbon atoms that will constitute the double bond all lie in a plane. This arrangement of atoms leads to syn elimination. Even though anti elimination is usually preferred, it does not occur for this compound because the bromine, hydrogen, and two carbons cannot achieve the necessary geometry. |
| 11.70 | Because Cl– is a relatively poor leaving group and acetate is a relatively poor nucleophile, a substitution reaction involving these two groups proceeds at a very slow rate. I–, however, is both a good nucleophile and a good leaving group. 1-Chlorooctane thus reacts preferentially with iodide to form 1-iodooctane. Only a small amount of 1-iodooctane is formed (because of the low concentration of iodide ion), but 1-iodooctane is more reactive than 1-chlorooctane toward substitution by acetate. Reaction with acetate produces 1-octyl acetate and regenerates iodide ion. The whole process can now be repeated with another molecule of 1-chlorooctane. The net result is production of 1-octyl acetate, and no iodide is consumed. |
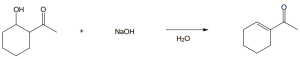
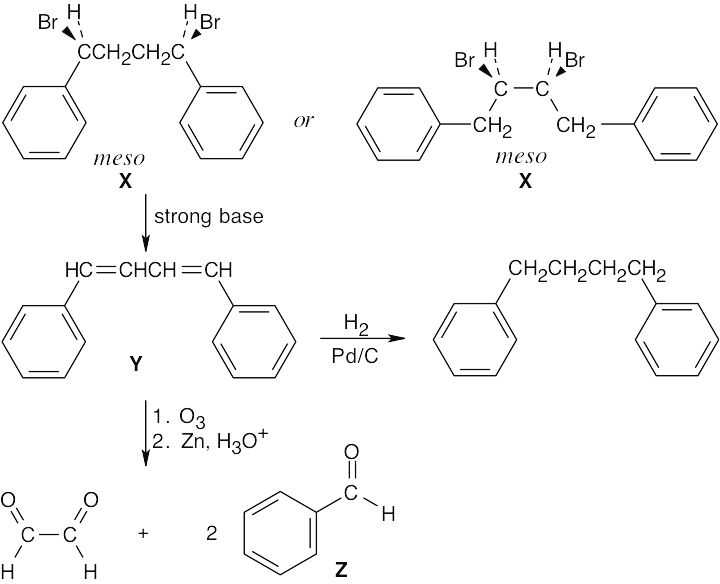
| 11.71 | Two optically inactive compounds are possible structures for compound X.
|
| 11.72 | 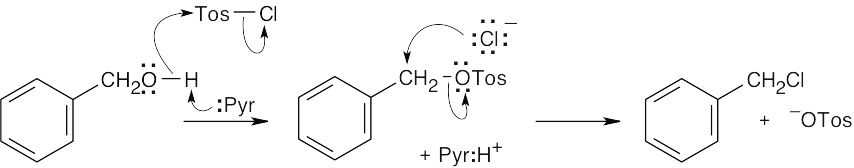
At lower temperatures, a tosylate is formed from the reaction of p-toluenesulfonyl chloride and an alcohol. The new bond is formed between the toluenesulfonyl group and the oxygen of the alcohol. At higher temperatures, the chloride anion can displace the –Otos group, which is an excellent leaving group, to form an organochloride. |
| 11.73 | Notice that the chiral methyl group has the (R) configuration in both N-methyltetrahydrofolate and in methionine. This fact suggests that methylation proceeds with two inversions of configuration which, in fact, has been shown to be the case. |
| 11.74 | 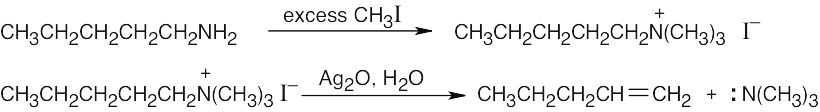
The intermediate is a charged quaternary ammonium compound that results from SN2 substitutions on three CH3I molecules by the amine nitrogen. E2 elimination occurs because the neutral N(CH3)3 molecule is a good leaving group. |
| 11.75 | (a) | 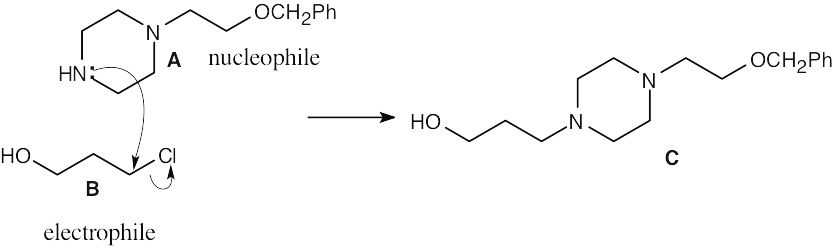 |
| (b) | 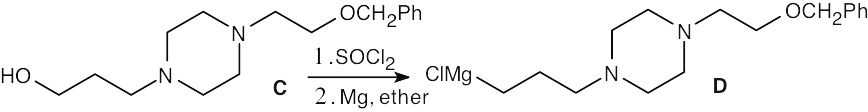 |
|
| (c) | 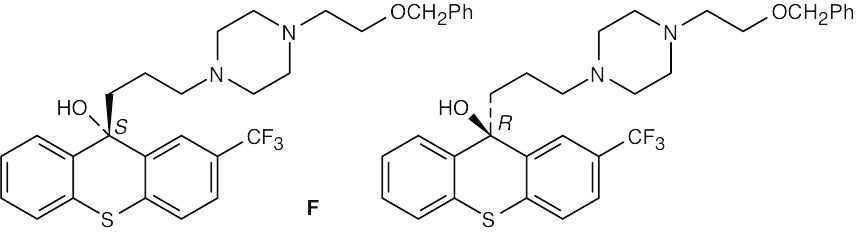
The stereoisomers are E,Z double bond isomers and are diastereomers. |
|
| (d) | 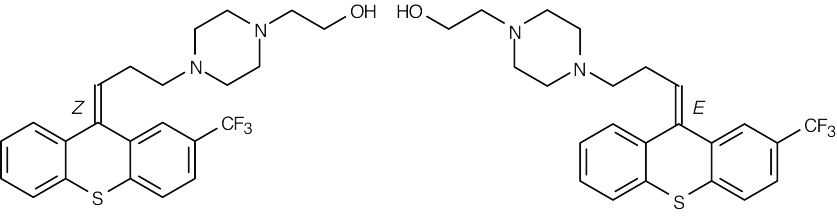 |
This file is copyright 2023, Rice University. All Rights Reserved.