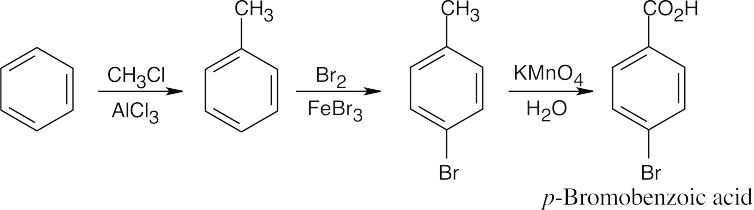
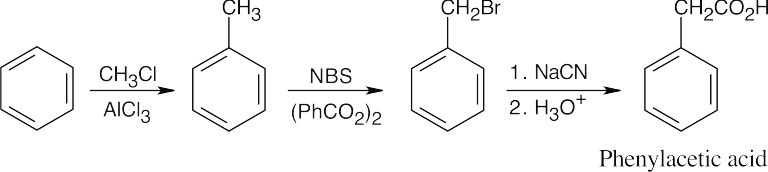
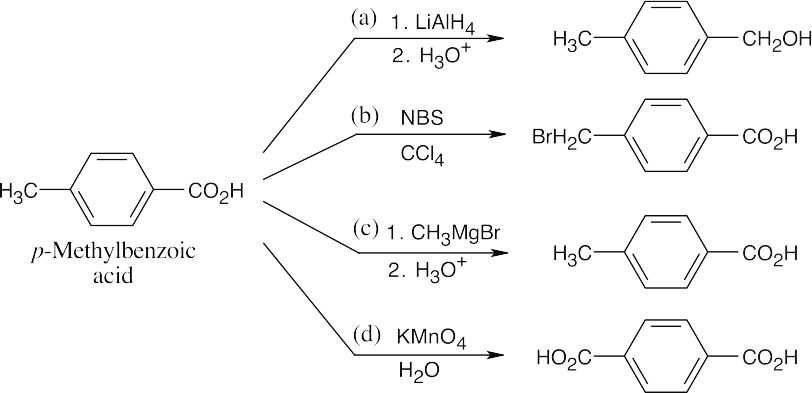
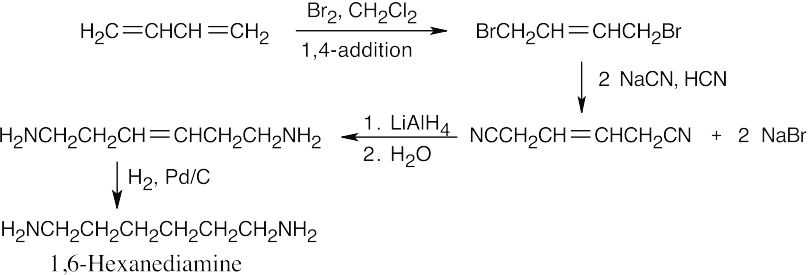
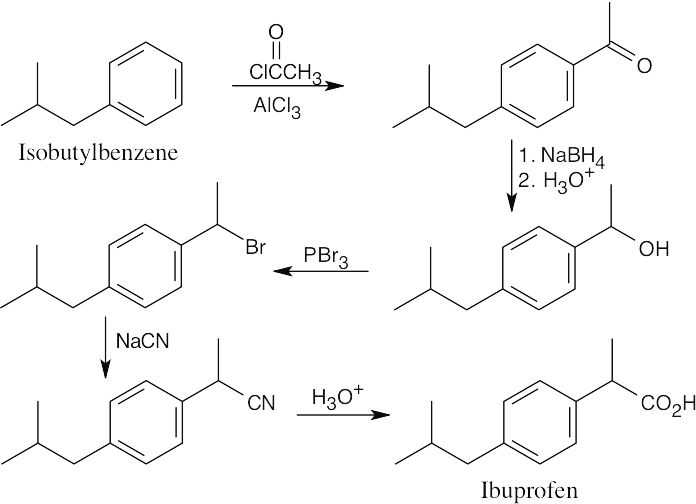
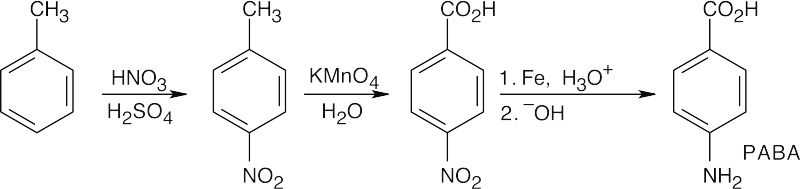
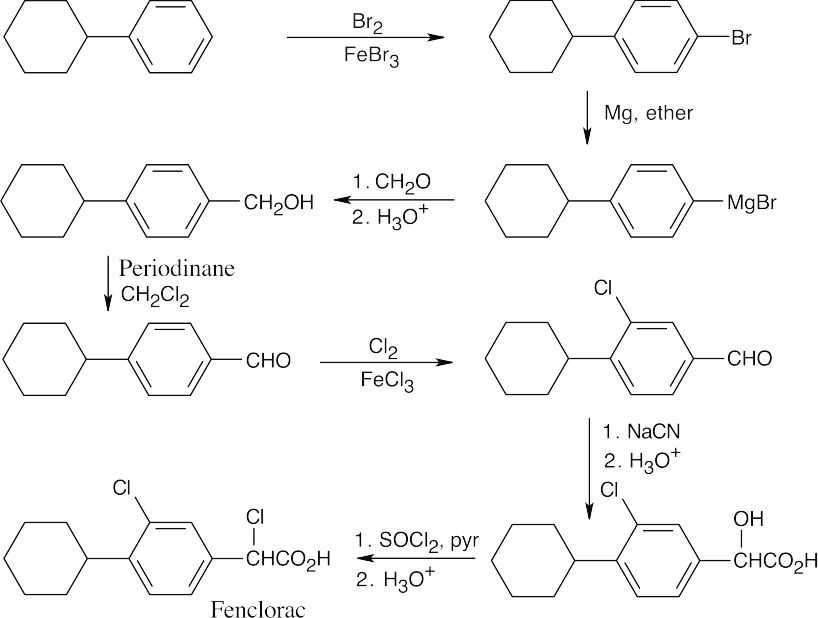
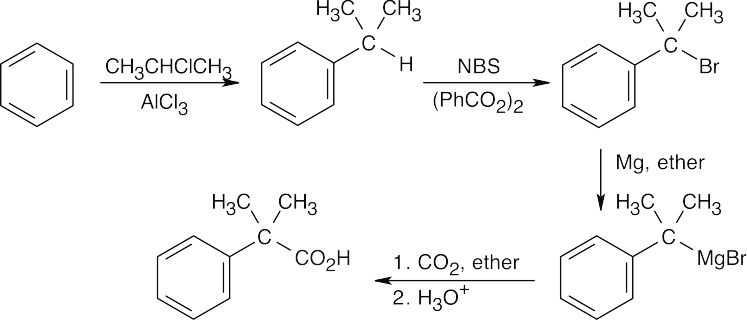
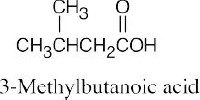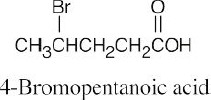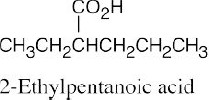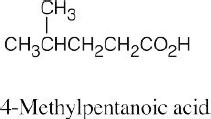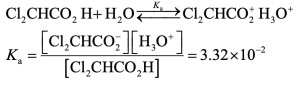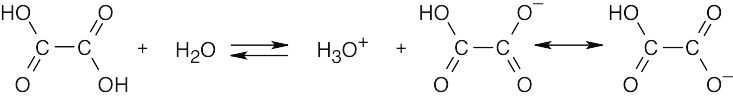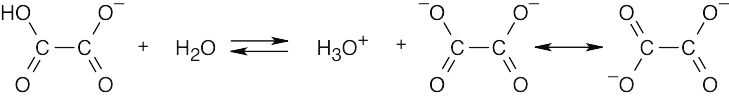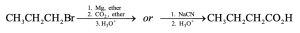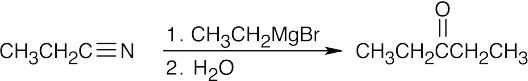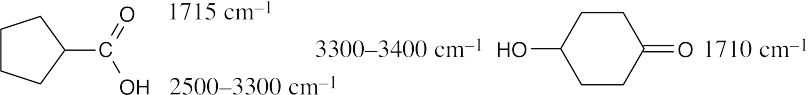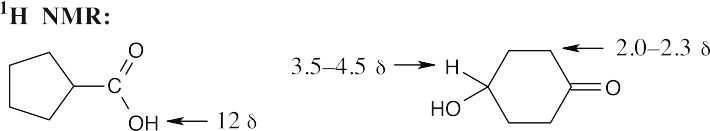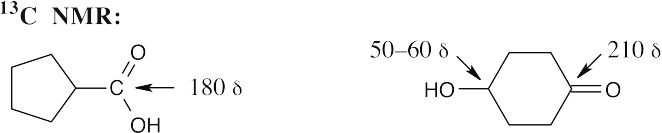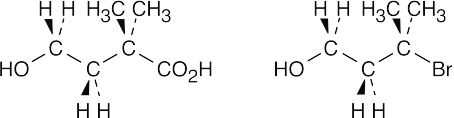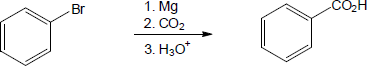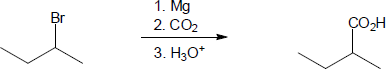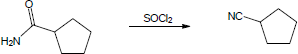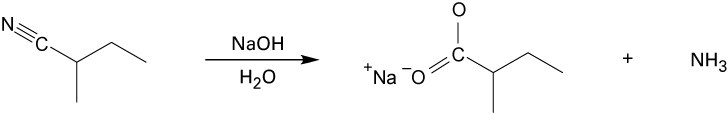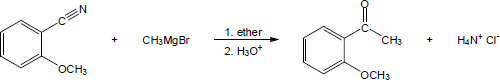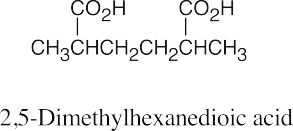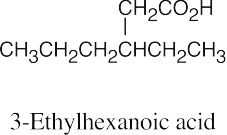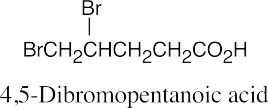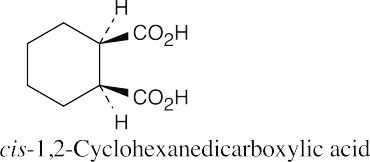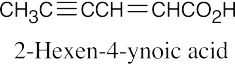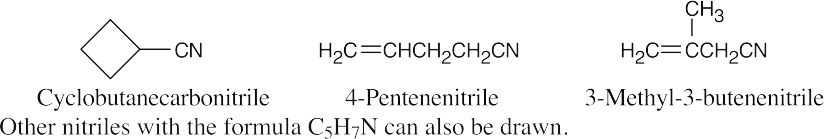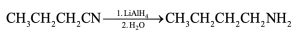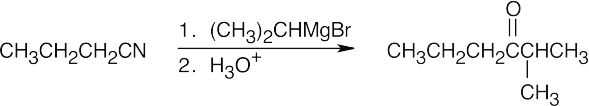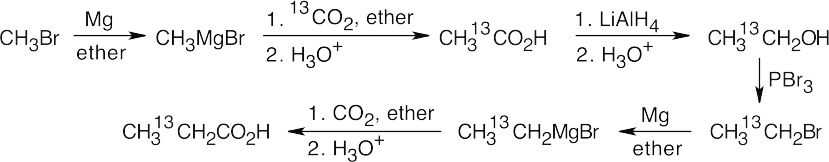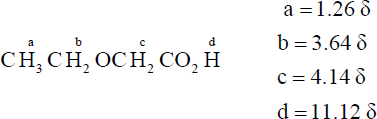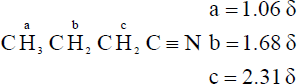20 Chapter 20 – Carboxylic Acids and Nitriles Solutions to Problems
Chapter 20 – Carboxylic Acids and Nitriles
Solutions to Problems
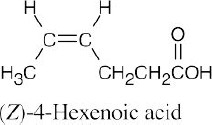
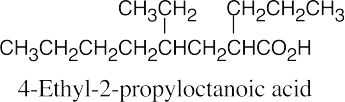
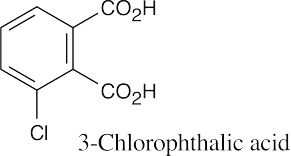
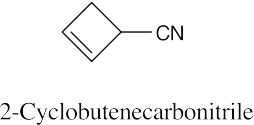
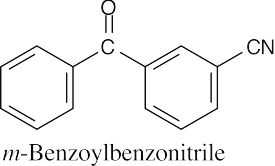
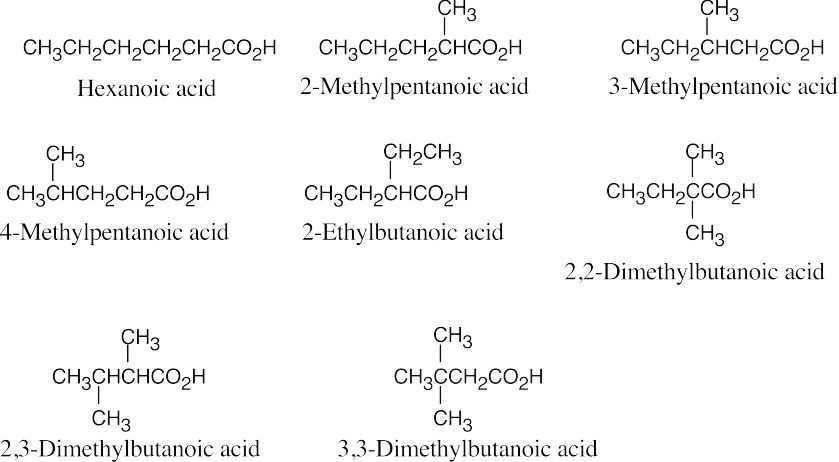
| 20.1 | Carboxylic acids are named by replacing –e of the corresponding alkane with –oic acid.
The carboxylic acid carbon is C1. When –CO2H is a substituent of a ring, the suffix –carboxylic acid is used; the carboxyl carbon is not numbered in this system.
|
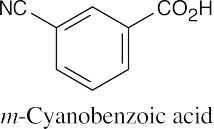
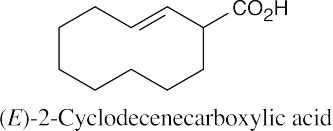
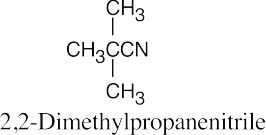
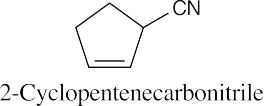
| 20.2 |
|
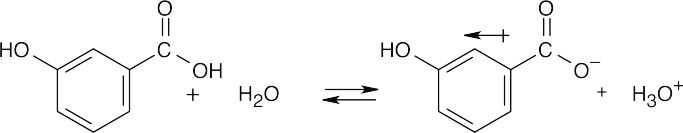
| 20.3 | Naphthalene is insoluble in water and benzoic acid is only slightly soluble. The salt of benzoic acid is very soluble in water, however, and we can take advantage of this solubility in separating naphthalene from benzoic acid.
Dissolve the mixture in an organic solvent, and extract with a dilute aqueous solution of sodium hydroxide or sodium bicarbonate, which will neutralize benzoic acid. Naphthalene remains in the organic layer, and all the benzoic acid, now converted to the benzoate salt, is in the aqueous layer. To recover benzoic acid, remove the aqueous layer, acidify it with dilute mineral acid, and extract with an organic solvent. |
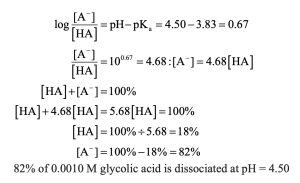
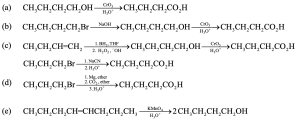
| 20.4 |
Using the quadratic formula to solve for y, we find that y = 0.0434 M
|
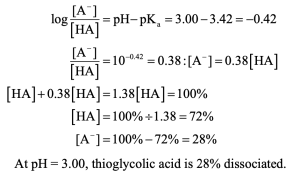
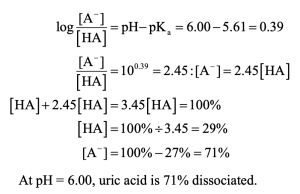
| 20.5 | Use the Henderson–Hasselbalch equation to calculate the ratio.
|
| 20.6 | You would expect lactic acid to be a stronger acid because the electron-withdrawing inductive effect of the hydroxyl group can stabilize the lactate anion. |
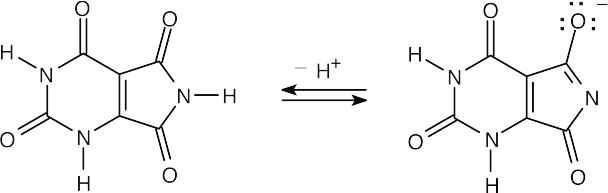
| 20.7 |
|
| 20.8 | A pKa of 4.45 indicates that p-cyclopropylbenzoic acid is a weaker acid than benzoic acid. This, in turn, indicates that a cyclopropyl group must be electron-donating. Since electron-donating groups increase reactivity in electrophilic substitution reactions, p-cyclopropylbenzene should be more reactive than benzene toward electrophilic bromination. |
| 20.9 | Remember that electron-withdrawing groups increase carboxylic acid acidity, and electron-donating groups decrease carboxylic acid acidity. Benzoic acid is more acidic than acetic acid.
|
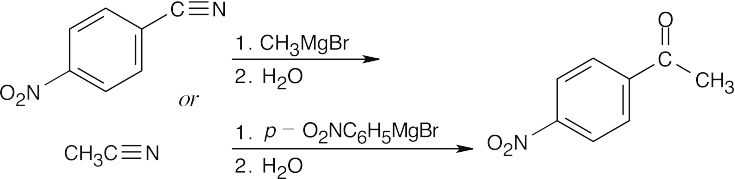
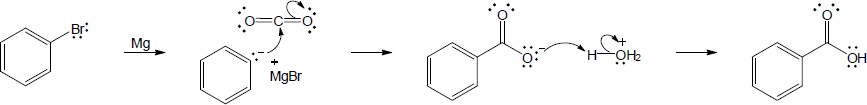
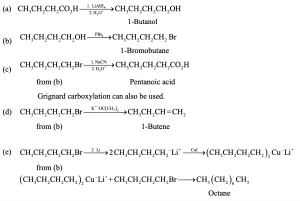
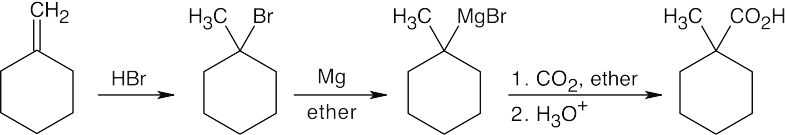
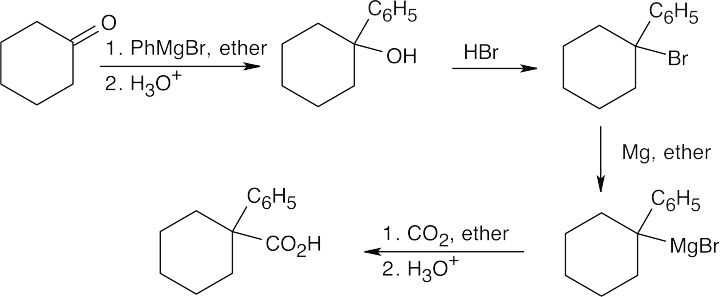
| 20.10 | In part (a), Grignard carboxylation must be used because the starting materials can’t undergo SN2 reactions. In (b), either method can be used.
|
| 20.11 |
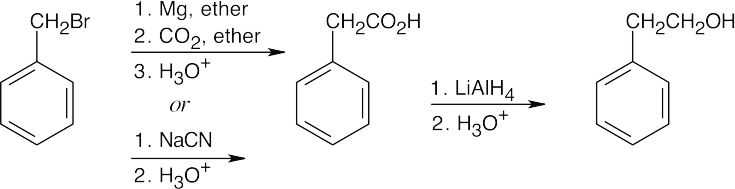
The alcohol product can be formed by reduction of a carboxylic acid with LiAlH4. The carboxylic acid can be synthesized either by Grignard carboxylation or by nitrile hydrolysis. The product can also be formed by a Grignard reaction between benzyl bromide and formaldehyde. |
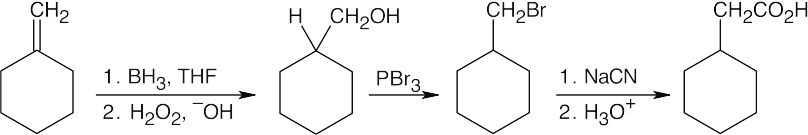
| 20.12 |
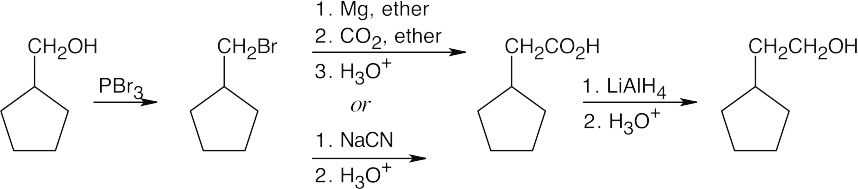
After treating the initial alcohol with PBr3, the same steps as used in the previous problem can be followed. A Grignard reaction between the cycloalkylmagnesium bromide and formaldehyde also yields the desired product. |
| 20.13 |
|
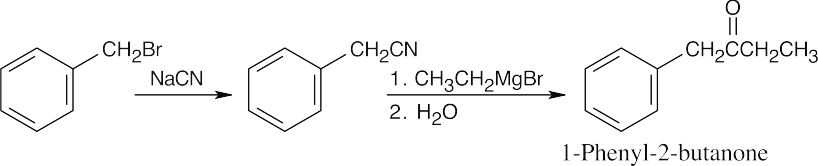
| 20.14 |
Once you realize that the product results from a Grignard reaction with a nitrile, this synthesis is easy. |
| 20.15 |
The positions of the carbonyl absorptions are too similar to be useful. The –OH absorptions, however, are sufficiently different for distinguishing between the compounds; the broad band of the carboxylic acid hydroxyl group is especially noticeable. |
| 10.16 |
|
Additional Problems
Visualizing Chemistry
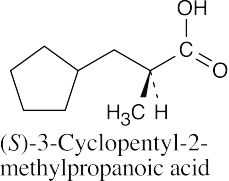
| 20.17 |
|
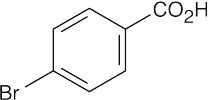
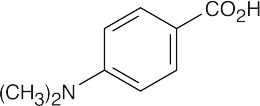
| 20.18 |
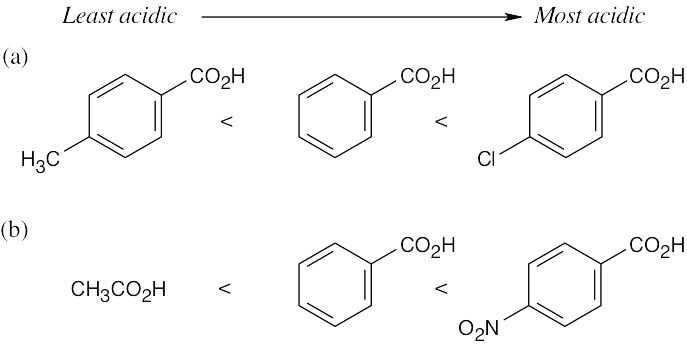
(a) p-Bromobenzoic acid is more acidic than benzoic acid because the electron-withdrawing bromine stabilizes the carboxylate anion. (b) This p-substituted aminobenzoic acid is less acidic than benzoic acid because the electron-donating group destabilizes the carboxylate anion. |
| 20.19 |
Nitrile hydrolysis can’t be used to synthesize the above carboxylic acid because the tertiary halide precursor (shown on the right) doesn’t undergo SN2 substitution with cyanide. Grignard carboxylation also can’t be used because the acidic hydroxyl hydrogen interferes with formation of the Grignard reagent. If the hydroxyl group is protected, however, Grignard carboxylation can take place. |
| 20.20 | The electrostatic potential maps show that the aromatic ring of anisole is more electron-rich (red) than the aromatic ring of thioanisole, indicating that the methoxyl group is more strongly electron-donating than the methylthio group. Since electron-donating groups decrease acidity, p-(methylthio)benzoic acid is likely to be a stronger acid than p-methoxybenzoic acid. |
Mechanism Problems
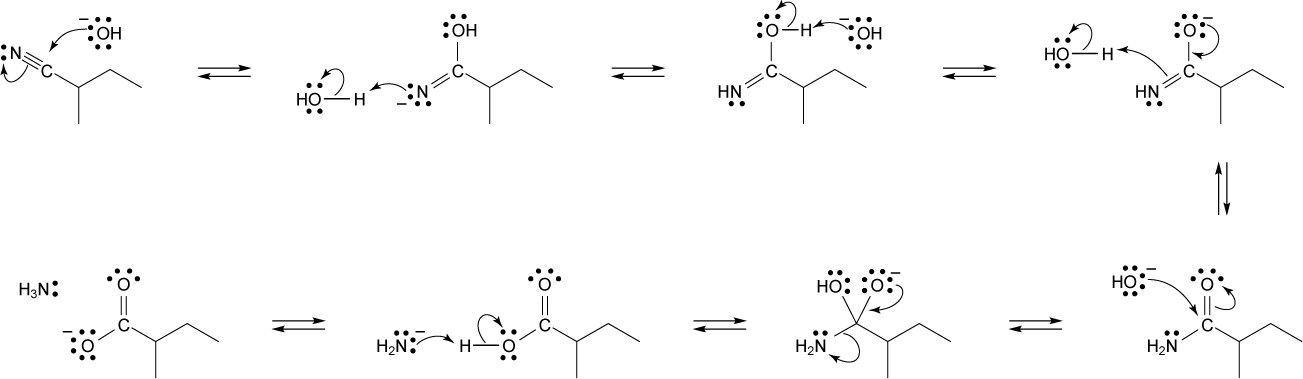
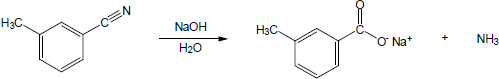
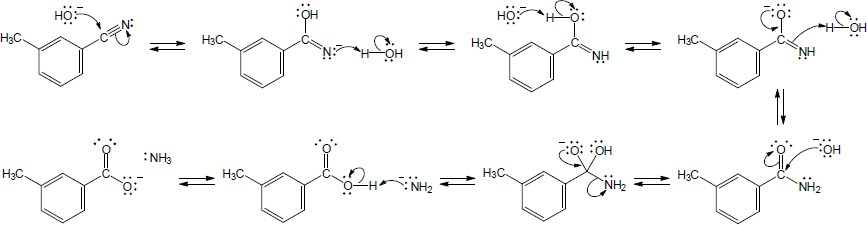
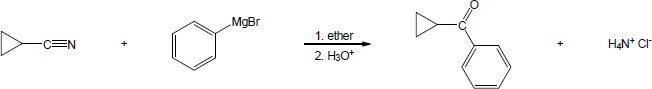
| 20.21 |
|
| 20.22 |
|
| 20.23 |
|
| 20.24 |
|
| 20.25 |
|
| 20.26 |
|
| 20.27 |
|
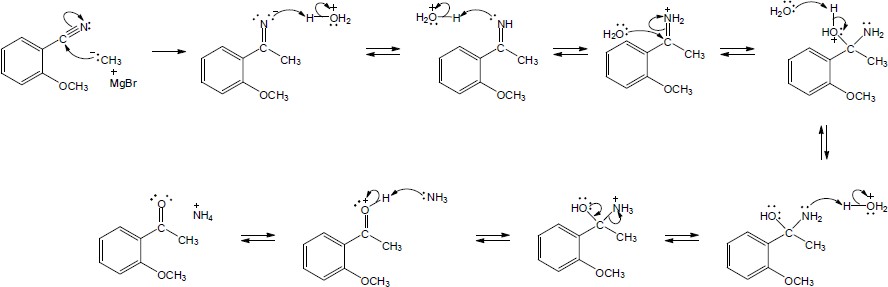
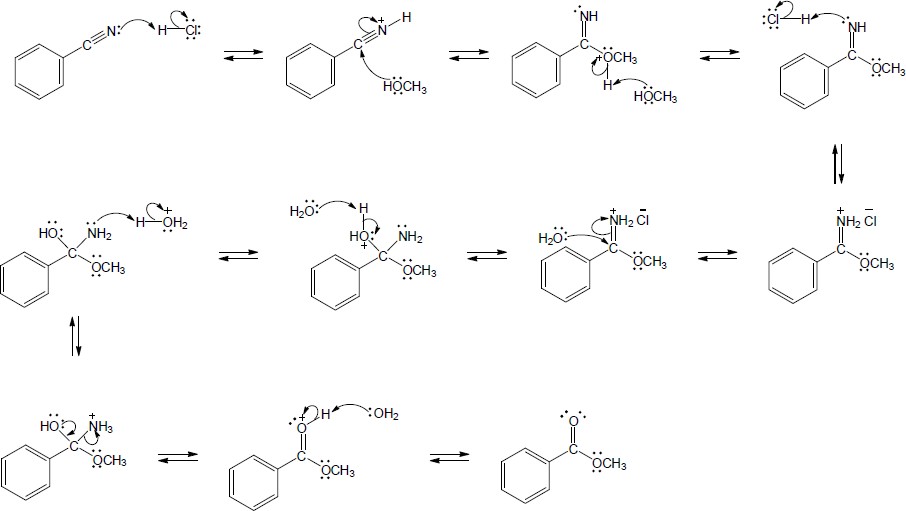
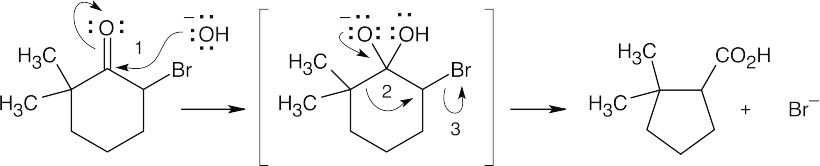
| 20.28 |
Nucleophilic addition (1), alkyl shift (2), and displacement of bromide (3) lead to the observed product. |
| 20.29 |
|
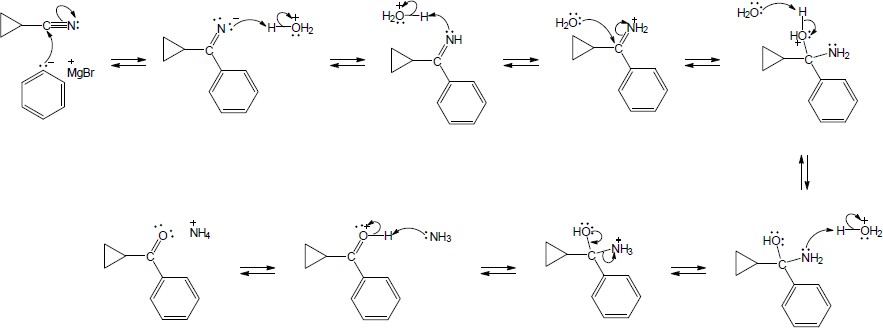
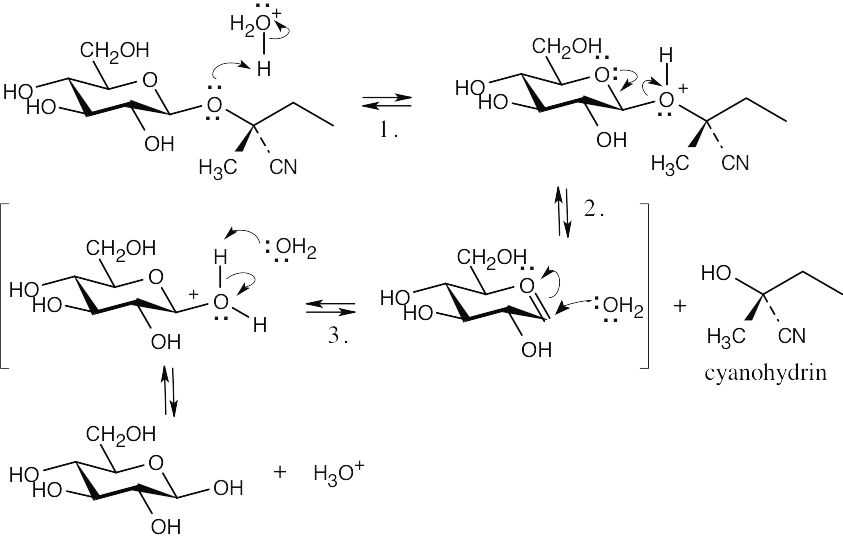
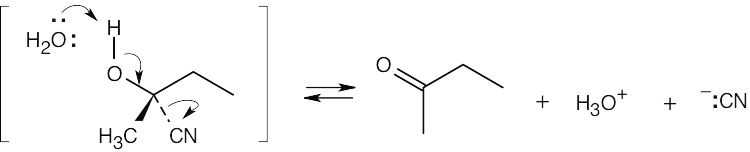
| 20.30 |
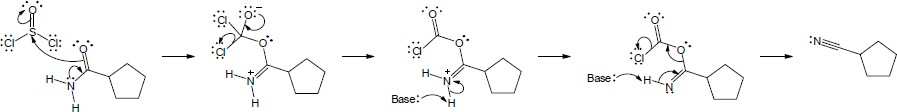
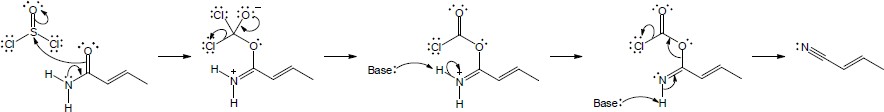
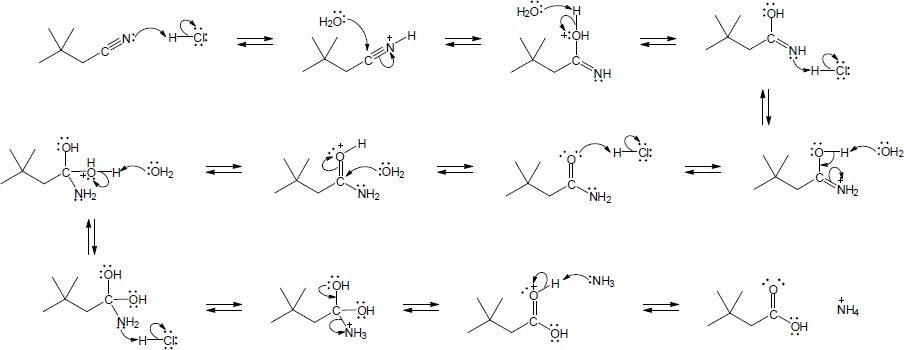
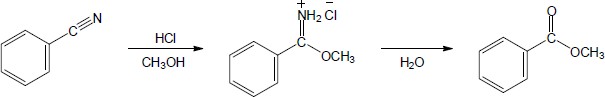
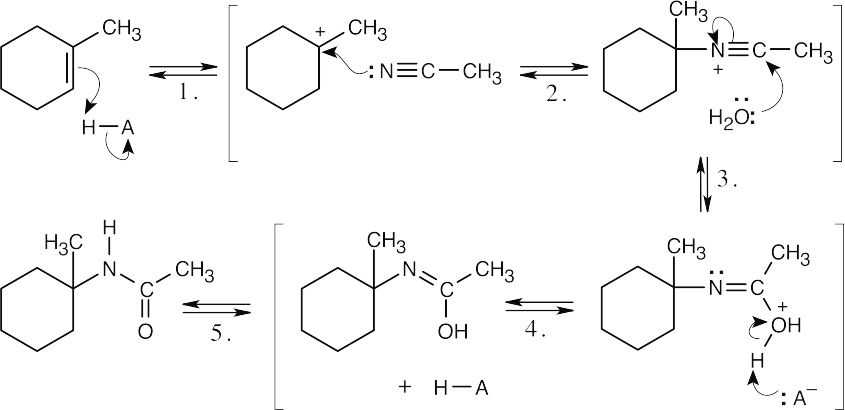
The following steps take place in the Ritter reaction: Step 1: Protonation of the alkene double bond; Step 2: Attack of the nitrogen lone pair electrons on the carbocation; Step 3: Attack of water on the nitrile carbon; Step 4: Deprotonation; Step 5: Tautomerization to the ketone. |
Naming Carboxylic Acids and Nitriles
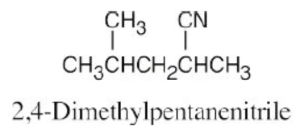
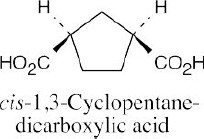
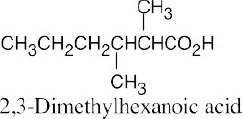
| 20.31 |
|
| 20.32 |
|
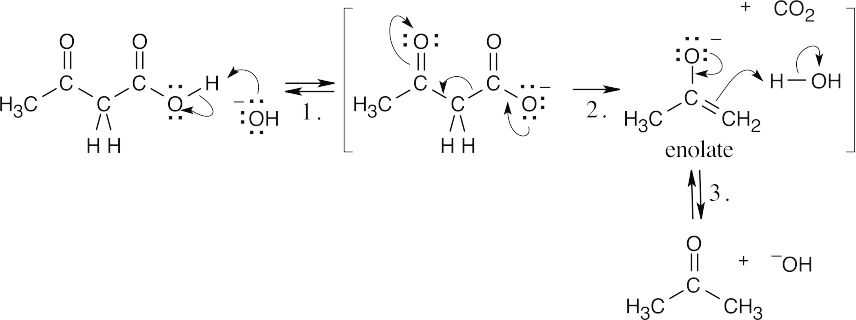
| 20.33 |
|
| 20.34 |
|
| 20.35 |
|
Acidity of Carboxylic Acids
| 20.36 | Less acidic ———-> More acidic
|
| 20.37 | Remember that the conjugate base of a weak acid is a strong base. In other words, the stronger the acid, the weaker the base derived from that acid.
Less basic —————> More basic
|
| 20.38 |
|
| 20.39 |
|
| 20.40 |
|
| 20.41 |
|
| 20.42 | Inductive effects of functional groups are transmitted through σ bonds. For oxalic acid, the electron-withdrawing inductive effect of one carboxylic acid group decreases the acidity of the remaining group. However, as the length of the carbon chain increases, the effect of one functional group on another decreases. In this example, the influence of the second carboxylic acid group on the ionization of the first is barely felt by succinic and adipic acids. |
Reactions of Carboxylic Acids and Nitriles
| 20.43 |
|
| 20.44 |
|
| 20.45 |
|
| 20.46 |
Alternatively, benzyl bromide can be converted to a Grignard reagent, poured over CO2, and the resulting mixture can be treated with aqueous acid in the last step. |
| 20.47 |
In (c), the acidic proton reacts with the Grignard reagent to form methane, for no net reaction. |
| 20.48 |
|
| 20.49 |
|
| 20.50 |
|
| 20.51 |
|
| 20.52 |
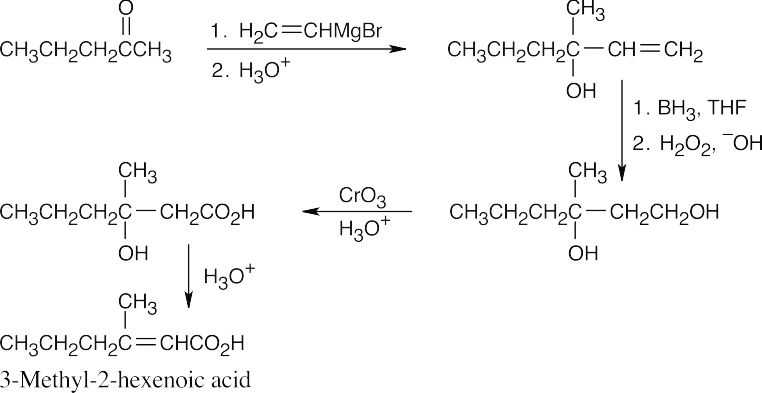
As in all of these more complex syntheses, other routes to the target compound are possible. This route was chosen because the Grignard reaction introduces a double bond without removing functionality at carbon 3. Dehydration occurs in the desired direction to produce a double bond conjugated with the carboxylic acid carbonyl group. |
Spectroscopy
| 20.53 | The peak at 1.08 δ is due to a tert-butyl group, and the peak at 11.2 δ is due to a carboxylic acid group. The compound is 3,3-dimethylbutanoic acid, (CH3)3CCH2CO2H. |
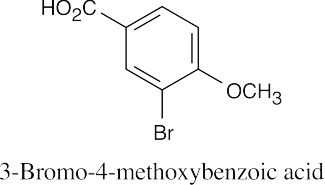
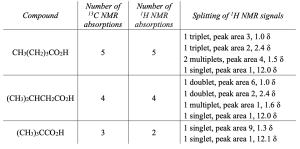
| 20.54 | Either 13C NMR or 1H NMR can be used to distinguish among these three isomeric carboxylic acids.
|
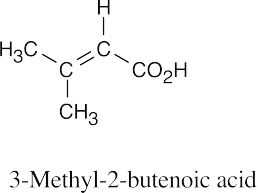
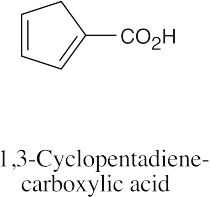
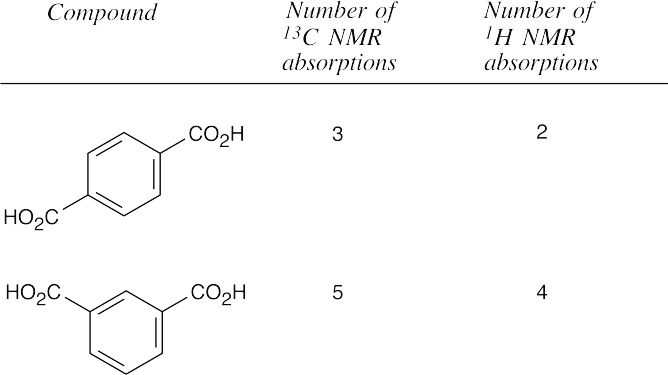
| 20.55 | In all of these pairs, different numbers of peaks occur in the spectra of each isomer. (a), (b) Use either 1H NMR or 13C NMR to distinguish between the isomers.
|
| 20.56 | The compound has one degree of unsaturation, which is due to the carboxylic acid absorption seen in the IR spectrum.
|
General Problems
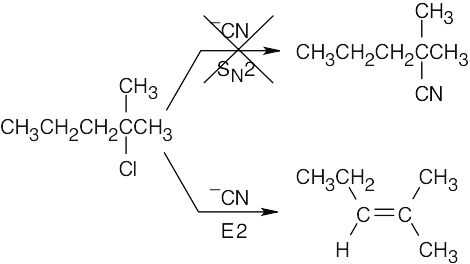
| 20.57 | 2-Chloro-2-methylpentane is a tertiary alkyl halide and –CN is a base. Instead of the desired SN2 reaction of cyanide with a halide, E2 elimination occurs and yields 2-methyl-2-pentene.
|
| 20.58 |
|
| 20.59 |
|
| 20.60 |
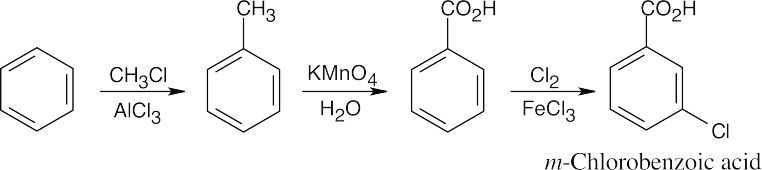
Notice that the order of the reactions is very important. If toluene is oxidized first, the nitro group will be introduced in the meta position. If the nitro group is reduced first, oxidation to the carboxylic acid will reoxidize the –NH2 group. |
| 20.61 |
Other routes to this compound are possible. The illustrated route was chosen because it introduced the potential benzylic functional group and the potential carboxylic acid in one step. Notice that the aldehyde functional group and the cyclohexyl group both serve to direct the aromatic chlorination to the correct position. Also, reaction of the hydroxy acid with SOCl2 converts –OH to –Cl and –CO2H to –COCl. Treatment with H3O+ regenerates the carboxylic acid. |
| 20.62 |
* Electrophilic aromatic substitution Recall from Section 20.4 that substituents that increase acidity also decrease reactivity in electrophilic aromatic substitution reactions. Of the above substituents, only –Si(CH3)3 is an activator. |
| 20.63 |
|
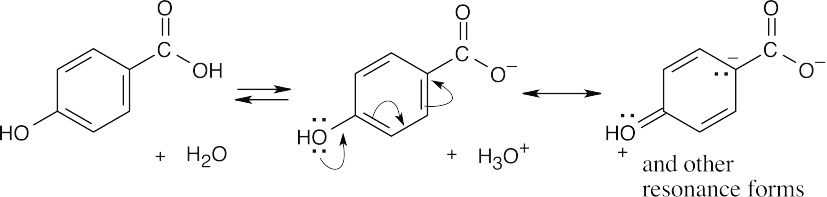
| 20.64 | As we have seen throughout this book, the influence of substituents on reactions can be due to inductive effects and/or resonance effects. For m-hydroxybenzoic acid, the negative charge of the carboxylate anion is stabilized by the electron-withdrawing inductive effect of –OH, making this isomer more acidic. For p-hydroxybenzoic acid, the negative charge of the anion is destabilized by the electron-donating resonance effect of –OH that acts over the π electron system of the ring but is not important for m-substituents.
|
| 20.65 |
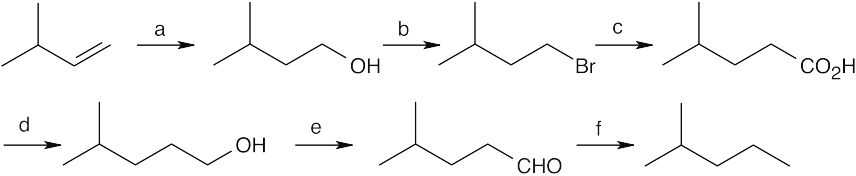
(a) BH3, THF, then H2O2, OH–; (b) PBr3; (c) Mg, then CO2, then H3O+ (or –CN, then H3O+); (d) LiAlH4, then H3O+; (e) Dess–Martin periodinane, CH2Cl2; (f) H2NNH2, KOH |
| 20.66 | A compound with the formula C4H7N has two degrees of unsaturation. The IR absorption at 2250 cm–1 identifies this compound as a nitrile.
|
| 20.67 | Both compounds contain four different kinds of protons (the H2C= protons are nonequivalent). The carboxylic acid proton absorptions are easy to identify; the other three absorptions in each spectrum are more complex.
It is possible to assign the spectra by studying the methyl group absorptions. The methyl group peak of crotonic acid is split into a doublet by the geminal (CH3CH=) proton, while the methyl group absorption of methacrylic acid is a singlet. The first spectrum (a) is that of crotonic acid, and the second spectrum (b) is that of methacrylic acid. |
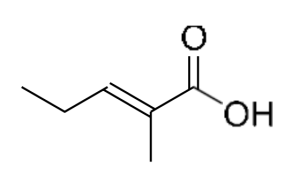
| 20.68 | 2-Methyl-2-pentenoic acid
|
| 20.69 |
|
| 20.70 |
Step 1: Deprotonation. Step 2: Decarboxylation. Step 3: Protonation. This reaction proceeds because of the loss of CO2 and the stability of the enolate anion. |