23 Chapter 23 – Carbonyl Condensation Reactions
Solutions to Problems
- (1)Form the enolate of one molecule of the carbonyl compound.
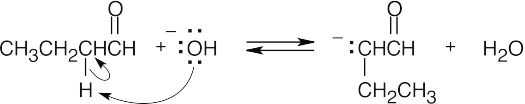
- Have the enolate attack the electrophilic carbonyl of the second molecule.
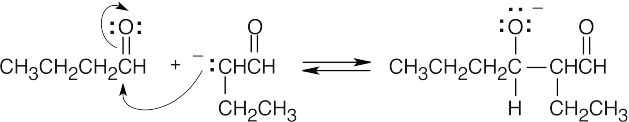
- Protonate the alkoxide oxygen.
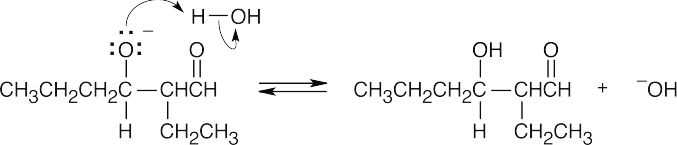
Practice writing out these steps for the other aldol condensations.
 See above. (b)
See above. (b)
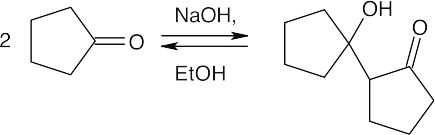 (c)
(c)
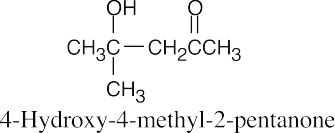
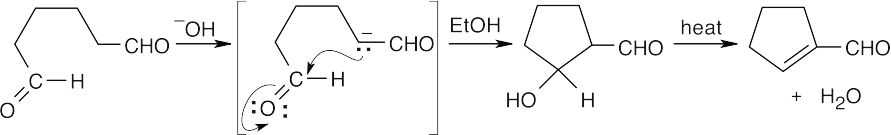
The steps for the reverse aldol are the reverse of those described in Problem 23.1.
- Deprotonate the alcohol oxygen.
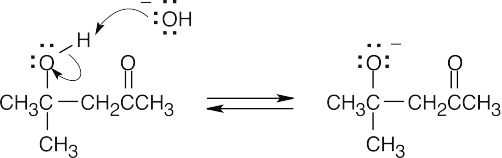
- Eliminate the enolate anion.
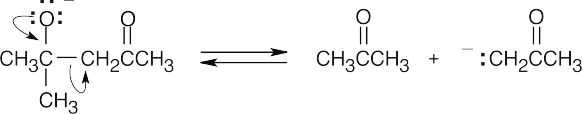
- Reprotonate the enolate anion.

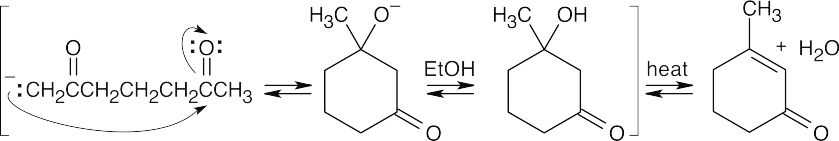
- As in Problem 23.1, align the two carbonyl compounds so that the location of the new bond is apparent. After drawing the addition product, form the conjugated enone product by dehydration. In parts (b) and (c), a mixture of E,Z isomers may be formed.
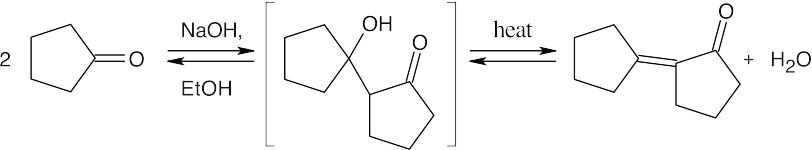 (a)
(a)
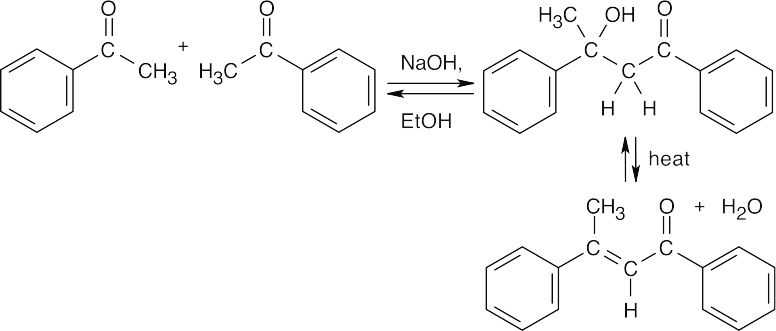 (b)
(b)
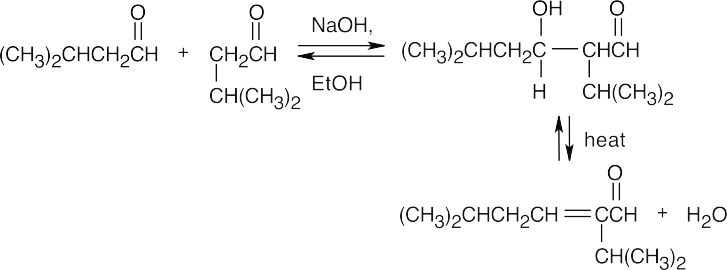 (c)
(c)
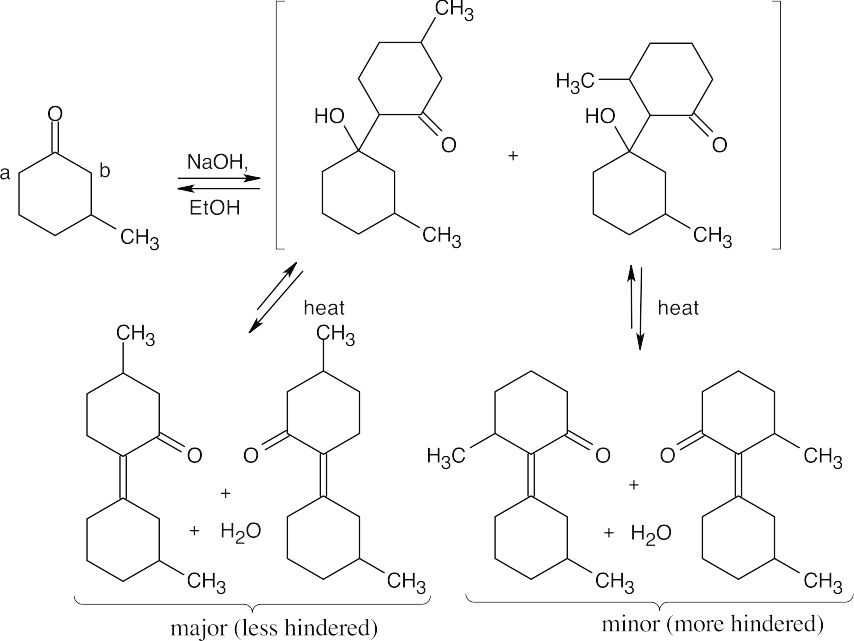
- Including double bond isomers, 4 products can be formed. The major product is formed by reaction of the enolate formed by abstraction of a proton at position “a” because position “b” has more steric hindrance.
23.5(a)
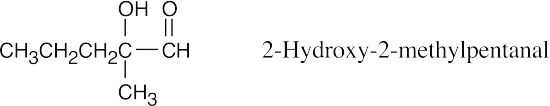
This is not an aldol product. The hydroxyl group in an aldol product must be β, not
α, to the carbonyl group.
(b)
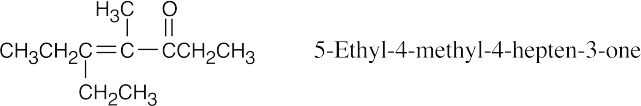
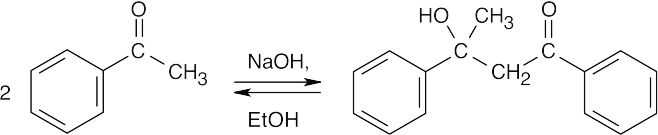
This product results from the aldol self-condensation of 3-pentanone, followed by dehydration.
23.6
23.7
23.8(a)
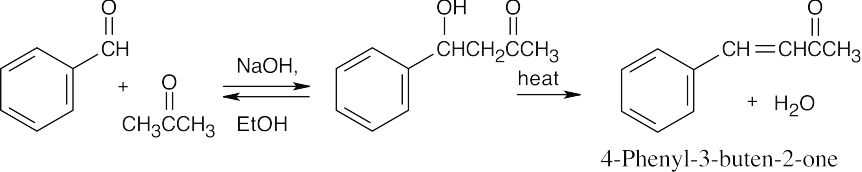
This mixed aldol will succeed because one of the components, benzaldehyde, is a good acceptor of nucleophiles, yet has no α-hydrogen atoms. Although it is possible for acetone to undergo self-condensation, the mixed aldol reaction is much more favorable.
 (b)
(b)
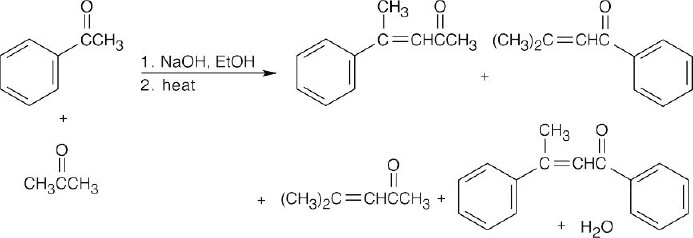
Four products result from the aldol condensation of acetone and acetophenone. The two upper compounds are mixed aldol products, and the bottom two are self- condensation products.
(c)
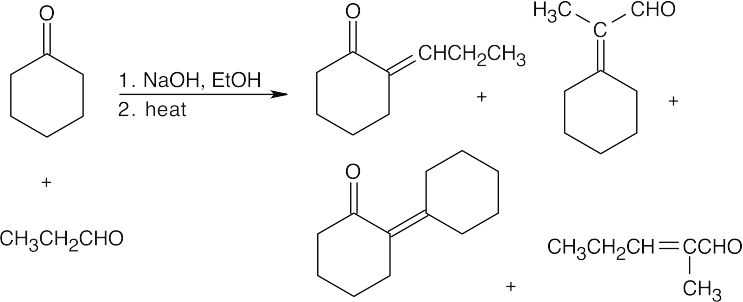
As in (b), a mixture of products is formed because both carbonyl partners contain α-hydrogen atoms. The upper two products result from mixed aldol condensations; the lower two are self-condensation products.
23.9
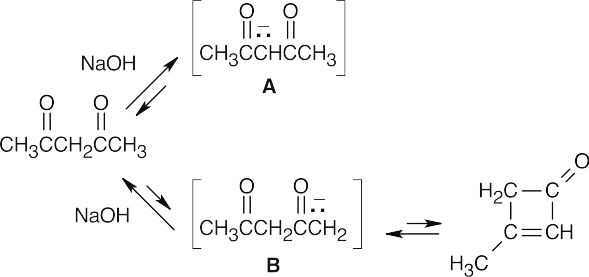
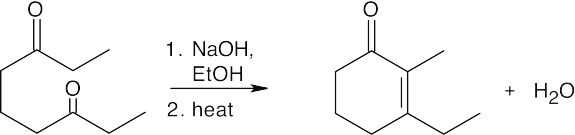
2,4–Pentanedione is in equilibrium with two enolate ions after treatment with base. Enolate A is stable and unreactive, while enolate B can undergo internal aldol condensation to form a cyclobutenone product. But, because the aldol reaction is reversible and the cyclobutenone product is highly strained, there is little of this product present when equilibrium is reached. At equilibrium, only the stable, diketone enolate ion A is present.
- This intramolecular aldol condensation gives a product with a seven-membered ring fused to a five-membered ring.
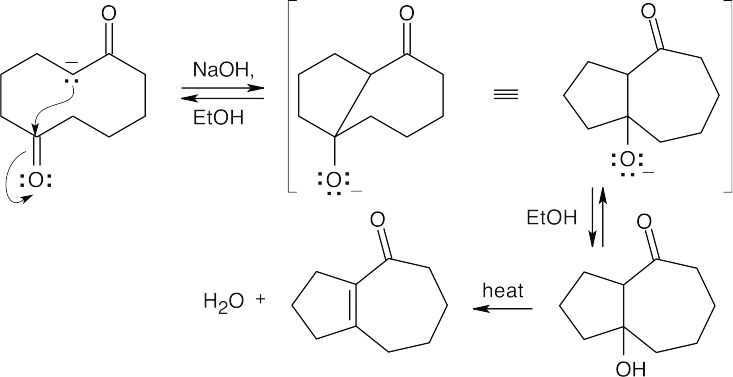
- As in the aldol condensation, writing the two Claisen components in the correct orientation makes it easier to predict the product.
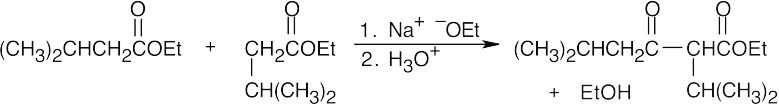 (a)
(a)
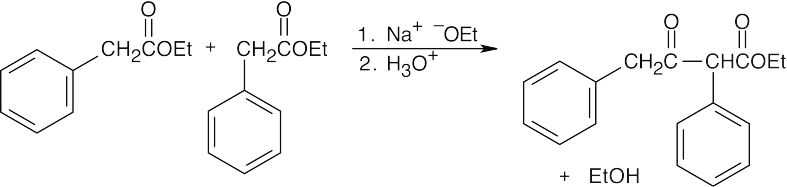 (b)
(b)
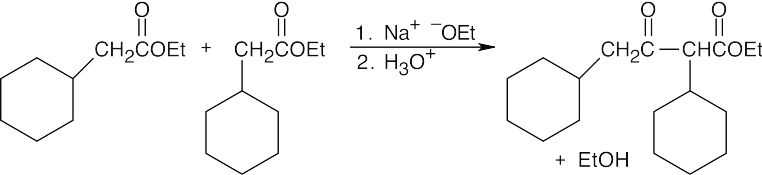 (c)
(c)
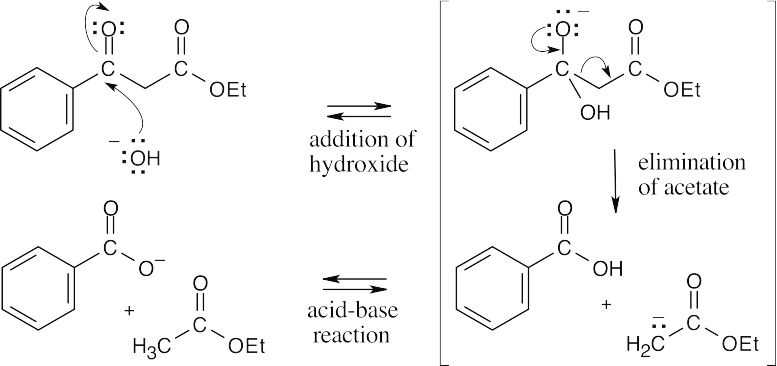
Hydroxide ion can react at two different sites of the β-keto ester. Abstraction of the acidic α-proton is more favorable but is reversible and does not lead to product. Addition of hydroxide ion to the carbon of the carbonyl group, followed by irreversible elimination of ethyl acetate anion, accounts for the observed product.
- As shown in Worked Example 23.4, dimethyl oxalate is a very effective reagent in mixed Claisen reactions.
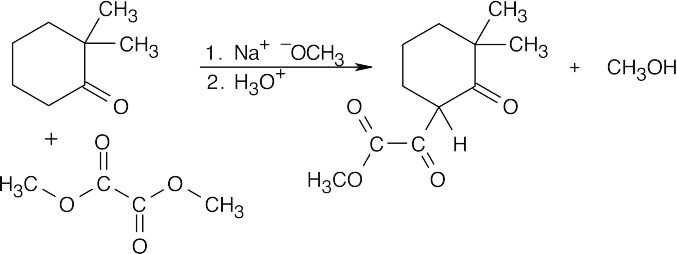
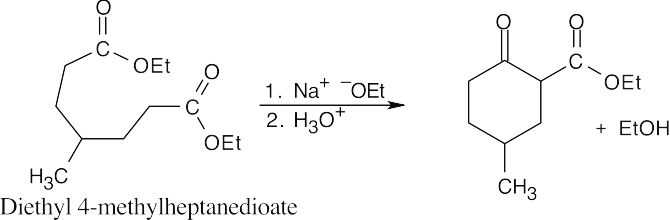
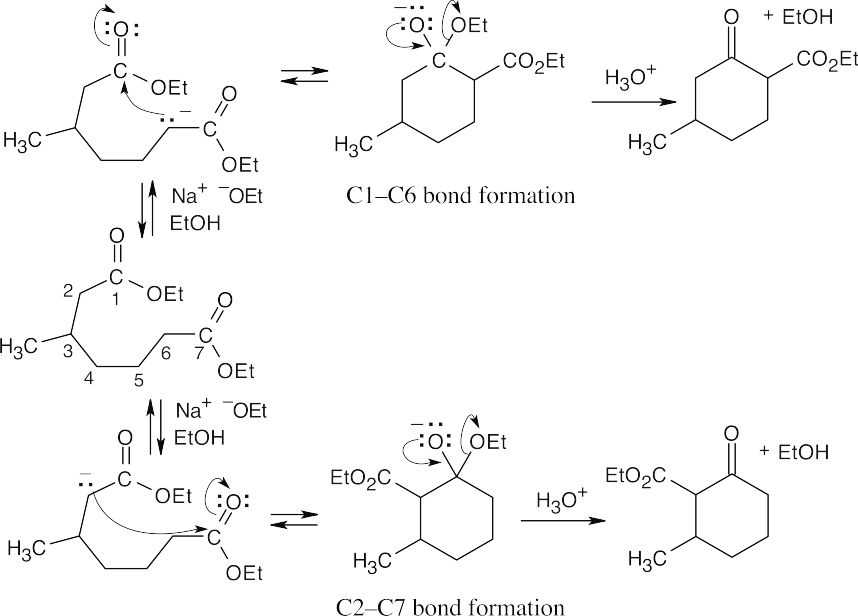
Unlike diethyl 4-methylheptanedioate, shown in the previous problem, diethyl 3- methylheptanedioate is unsymmetrical. Two different enolates can form, and each can cyclize to a different product.
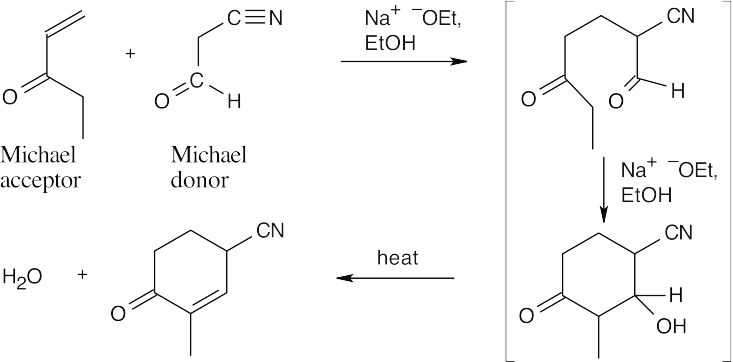
- A Michael reaction takes place when a stable enolate ion (Michael donor) adds to the double bond of an α,β-unsaturated carbonyl compound (Michael acceptor). The enolate adds to the double bond of the conjugated system. Predicting Michael products is easier when the donor and acceptor are positioned so that the product can be visualized.
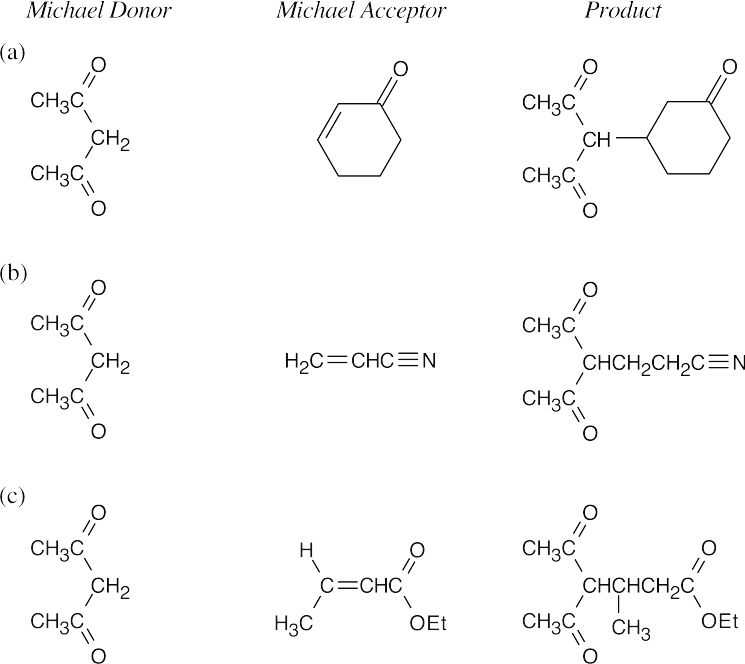
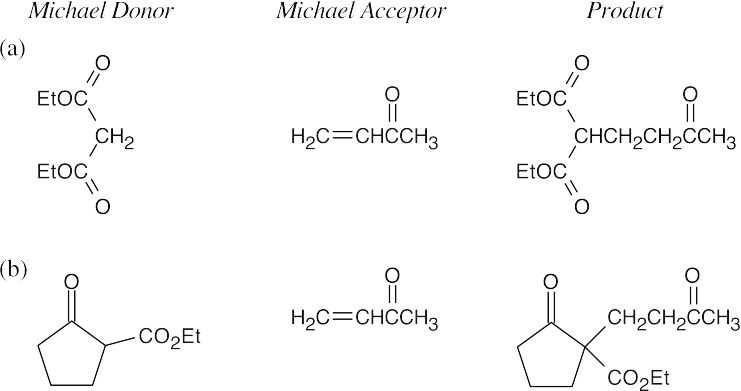
- Find the carbonyl group of the Michael acceptor, and count three carbons away from the carbonyl group. The Michael donor forms the new bond to this carbon. Break this bond to identify the Michael donor and Michael acceptor.
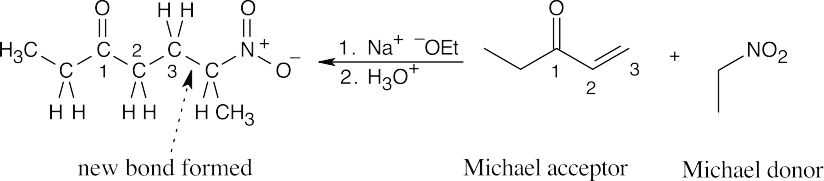
- An enamine is formed from a ketone when it is necessary to synthesize a 1,5-diketone or a 1,5-dicarbonyl compound containing an aldehyde or ketone. The ketone starting material is converted to an enamine in order to increase the reactivity of the ketone and to direct the regiochemistry of addition. The process, as described in Section 23.11, is: (1) conversion of a ketone to its enamine; (2) Michael addition to an α,β-unsaturated carbonyl compound; (3) hydrolysis of the substituted enamine to the diketone product.
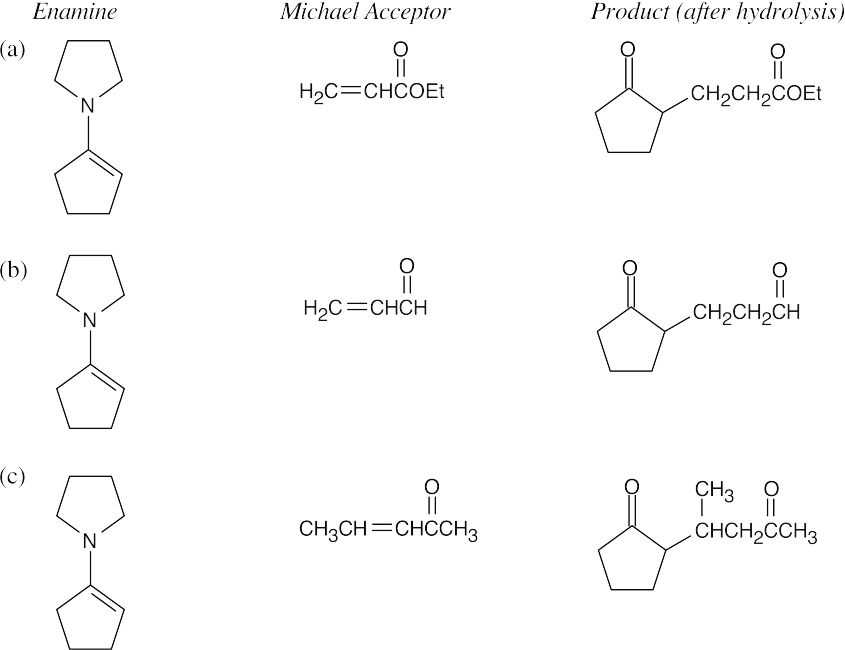
- Analyze the product for the Michael acceptor and the ketone. In (a), the Michael acceptor is propenenitrile. The ketone is cyclopentanone, which is treated with pyrrolidine to form the enamine.
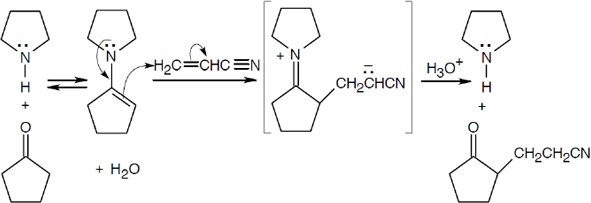 (a)
(a)
(b)
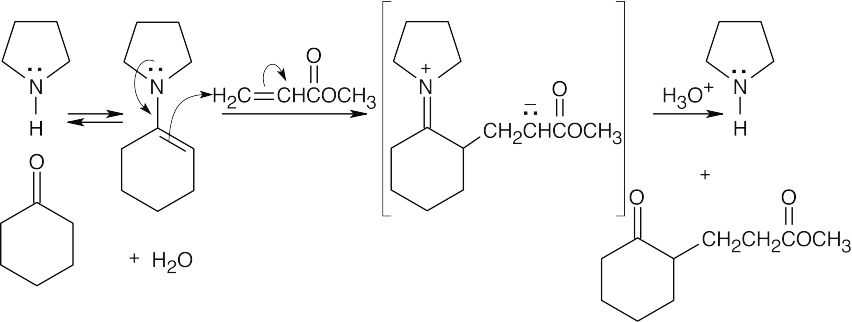
23.21 The Robinson annulation is a combination of two reactions covered in this chapter. First, a Michael reaction takes place between a nucleophilic donor (the diketone in this problem) and an α,β-unsaturated carbonyl compound (the enone shown). The resulting product can cyclize in an aldol reaction. The base catalyzes both reactions.
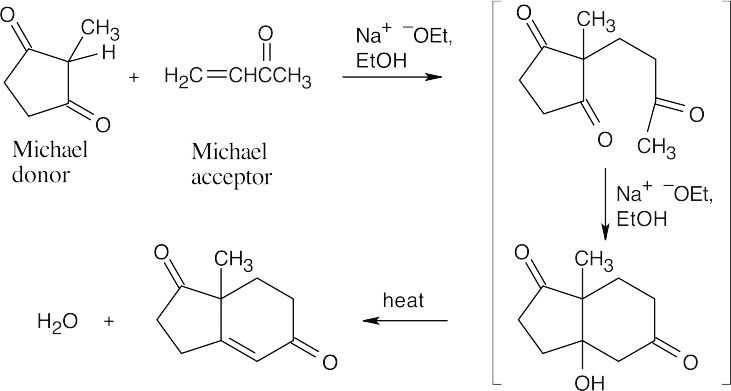
23.22
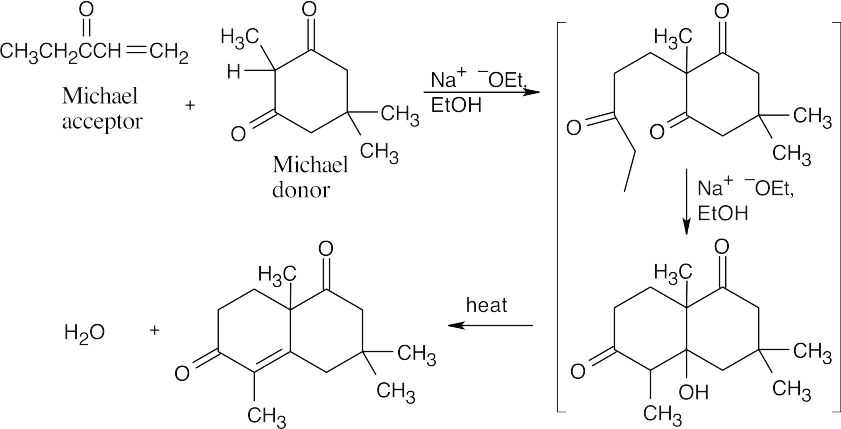
This is one of the more complicated-looking syntheses that we have seen. First, analyze the product for the two Michael components. The carbon–carbon double bond arises from dehydration of the aldol addition product, and is located where one of the two C=O groups of the original diketone used to be. The Michael addition takes place at the carbon between these ketone groups. The Michael acceptor is an enone that can also enter into the aldol condensation and furnishes the methyl group attached to the double bond.
Additional Problems
Visualizing Chemistry
23.23
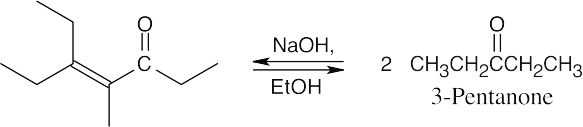 (a)
(a)
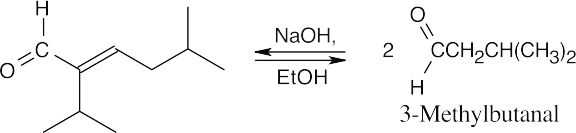 (b)
(b)
23.24 The enolate of methyl phenylacetate adds to a second molecule of methyl phenylacetate to form the Claisen intermediate that is pictured. Elimination of methoxide (circled) and acidification give the product shown.
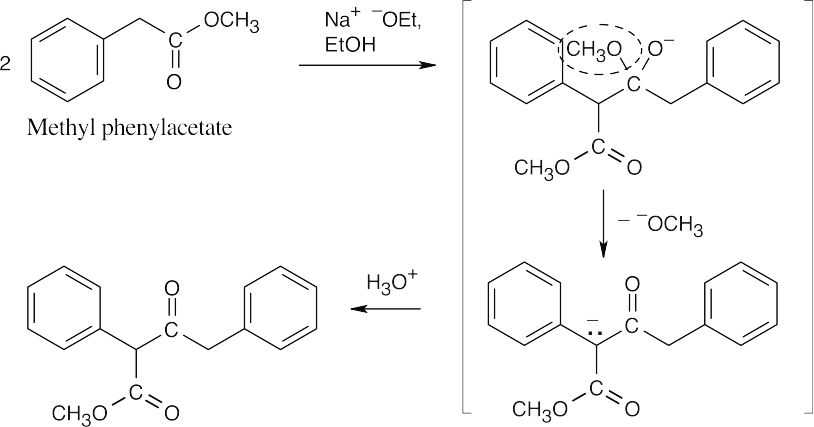
23.25
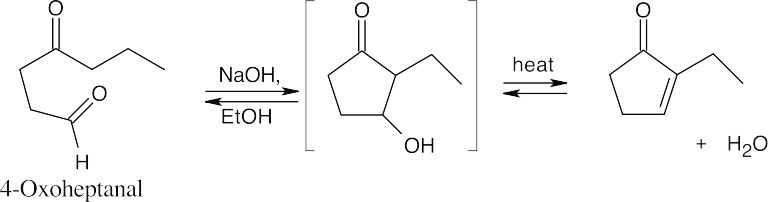
23.26 Remember that the new carbon–carbon double bond in the product connects one of the carbonyl carbons of the Michael donor with an α carbon of the Michael acceptor.
Mechanism Problems
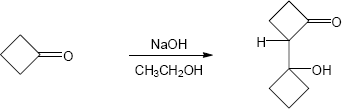 23.27 (a)
23.27 (a)
Mechanism:
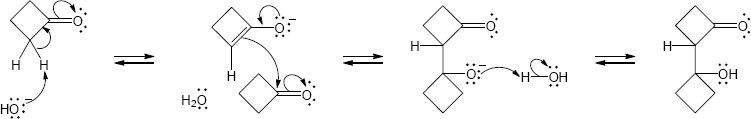
 (b)
(b)
 Mechanism:
Mechanism:
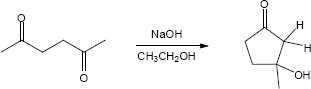
 23.28 (a)
23.28 (a)
Mechanism:
(b)
Mechanism:
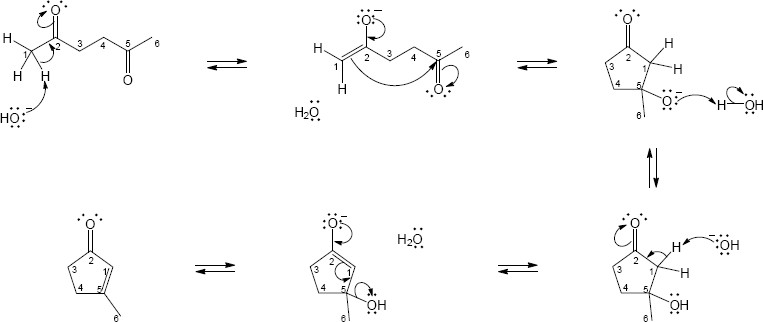
23.29 (a)
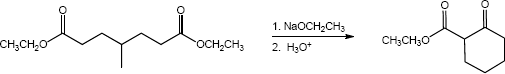
Mechanism:
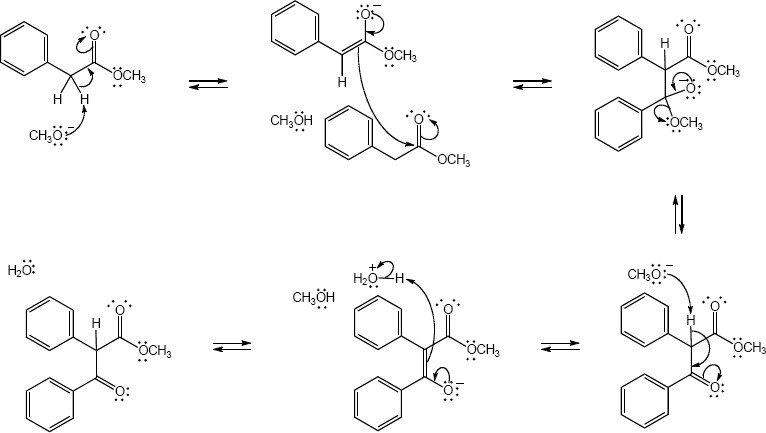 Mechanism:
Mechanism:
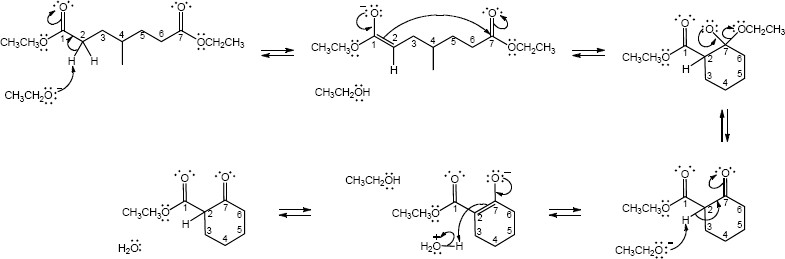
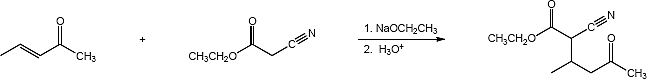 23.30 (a)
23.30 (a)
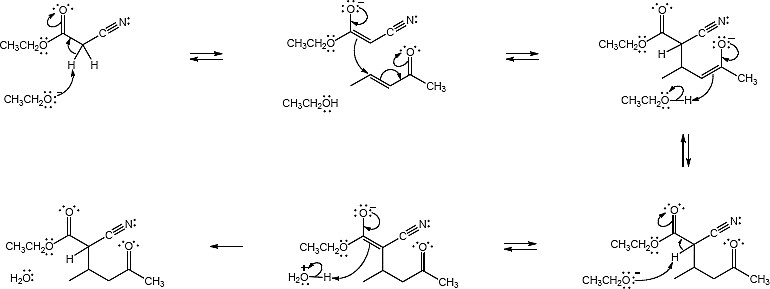 Mechanism:
Mechanism:
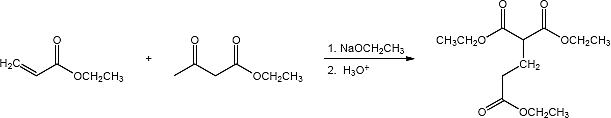 (b)
(b)
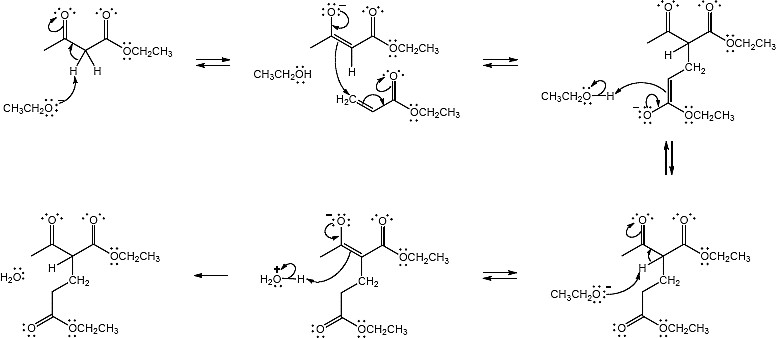 Mechanism:
Mechanism:
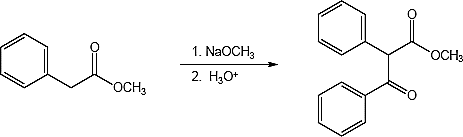
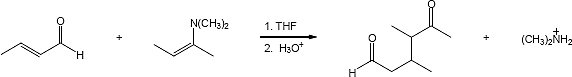 23.31 (a)
23.31 (a)
Mechanism:
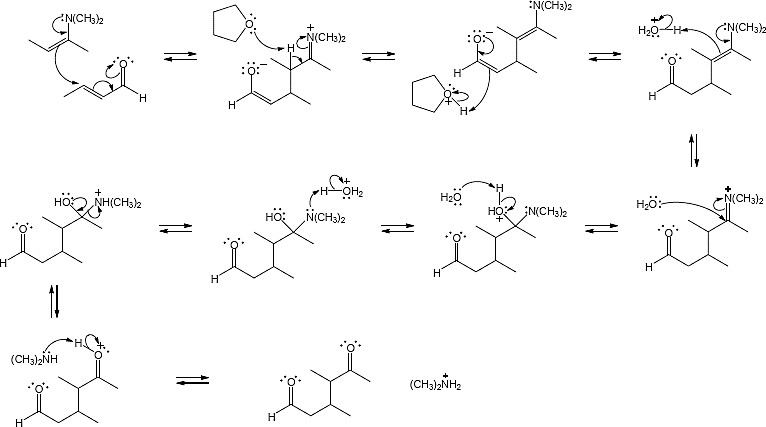
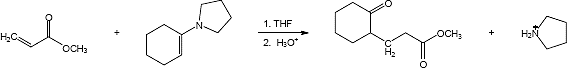
Mechanism:
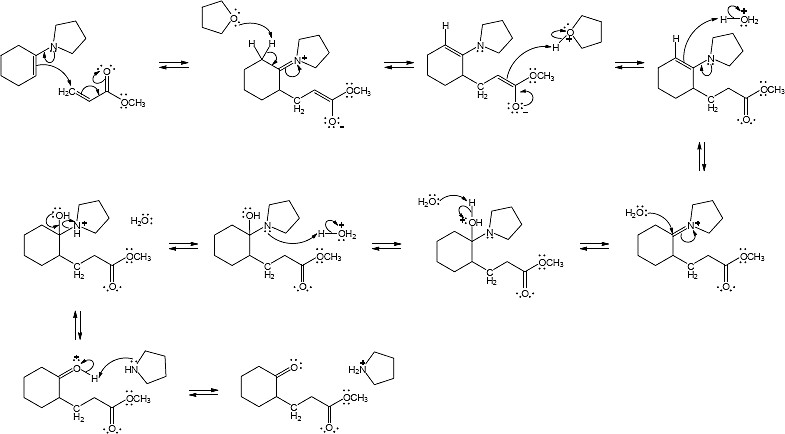
 23.32
23.32
Mechanism:
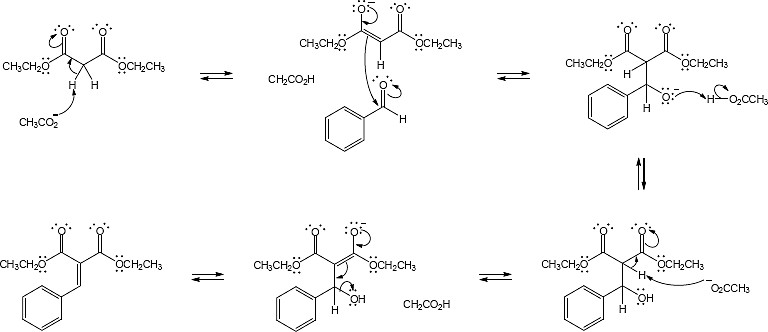
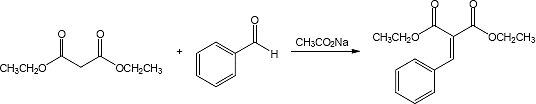
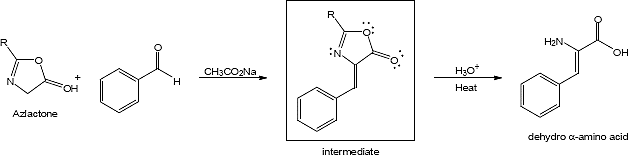 23.33
23.33
Mechanism:
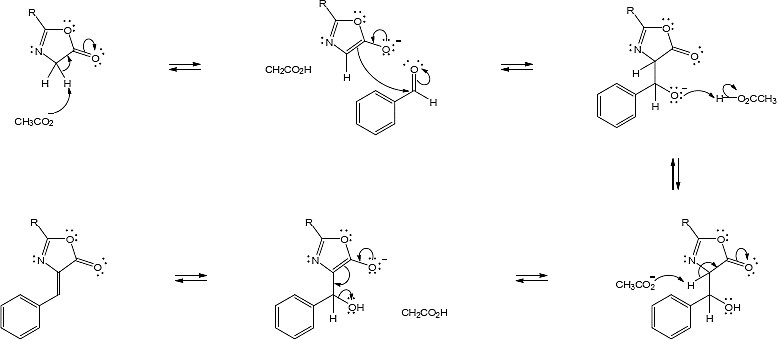
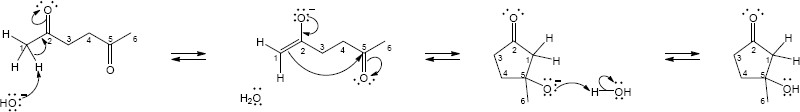
- This sequence is a reverse aldol reaction.
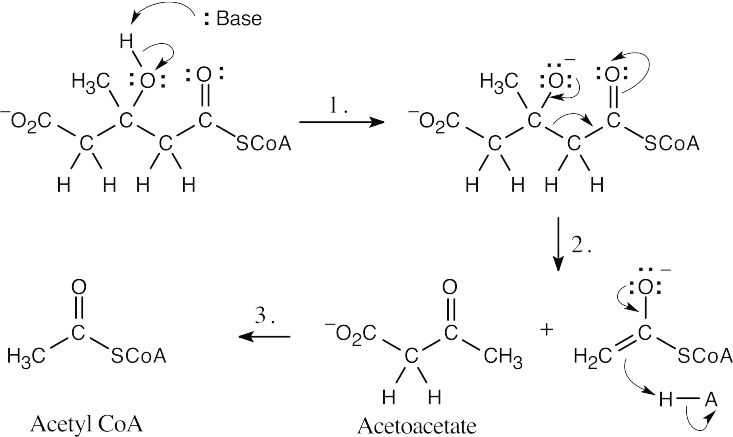
Step 1:Deprotonation by base.
Step 2:Elimination of acetyl CoA enolate.
Step 3:Protonation of enolate.
- In contrast to the previous problem, this sequence is a reverse Claisen reaction. The first step (not illustrated) is the reaction of HSCoA with a base to form –SCoA.
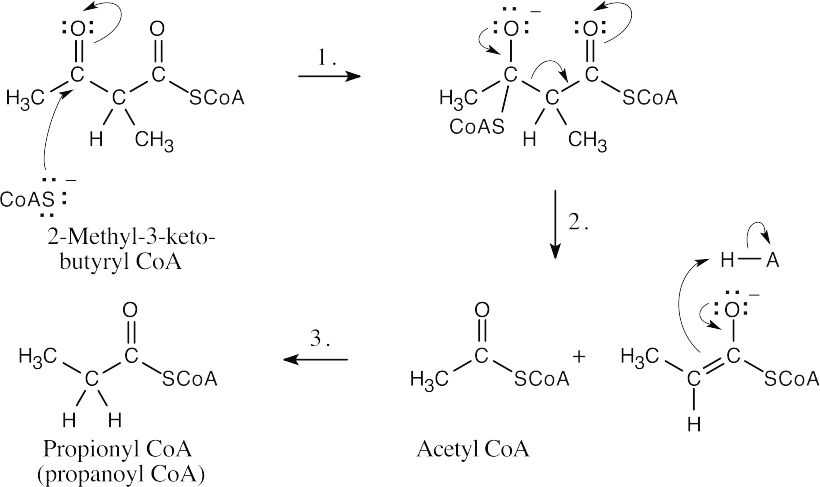
Step 1:Addition of –SCoA to form a tetrahedral intermediate.
Step 2:Elimination of propionyl CoA anion.
Step 3:Protonation.
Formation of (S)-citryl CoA:
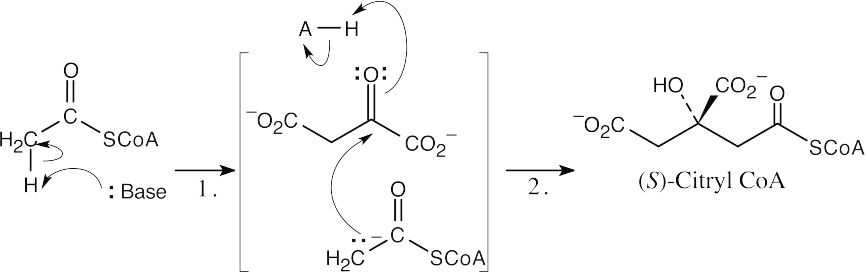
Step 1:Formation of acetyl CoA enolate.
Step 2:Aldol-like nucleophilic addition of acetyl CoA to the carbonyl group of oxaloacetate and protonation.
Loss of CoA to form citrate:
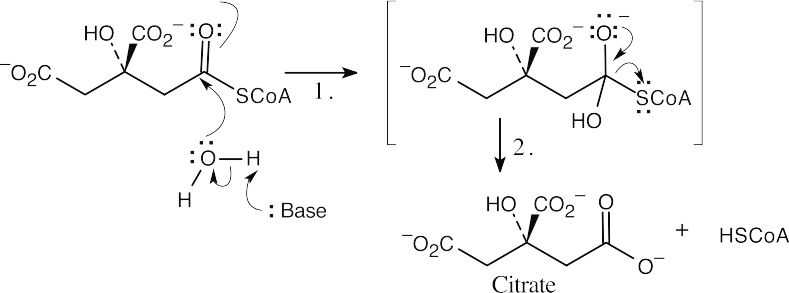
Step 1:Nucleophilic addition of hydroxyl to the carbonyl group of (S)-citryl CoA.
Step 2:Loss of –SCoA and protonation of the leaving group.
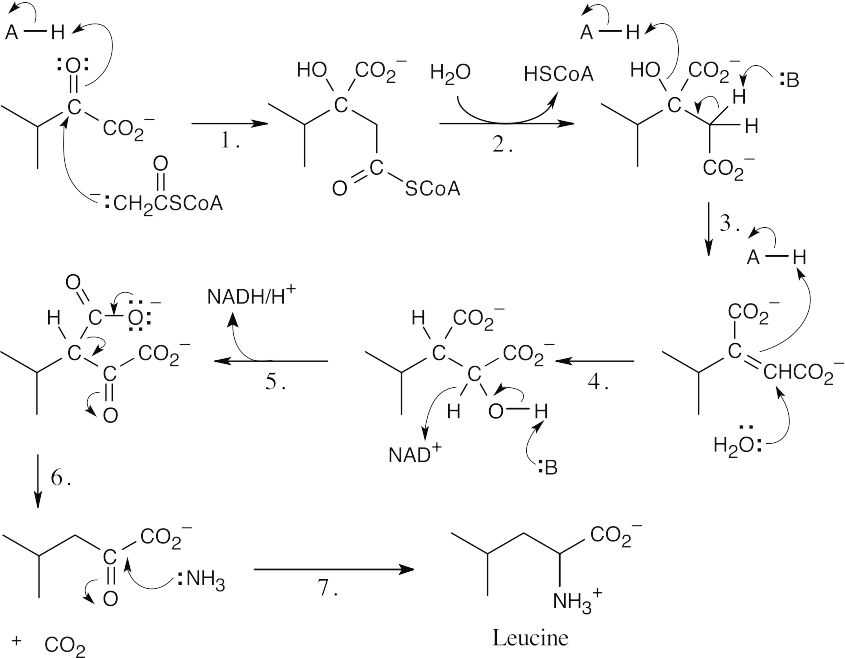
Step 1:Addition of –SCoA.
Step 2:Hydrolysis.
Step 3:Elimination.
Step 4:Conjugate addition.
Step 5:Oxidation.
Step 6:Decarboxylation of a β-keto ester.
Step 7:Nucleophilic acyl substitution, loss of water, reduction.
- Formation of the enolate of diethyl malonate is the first step:
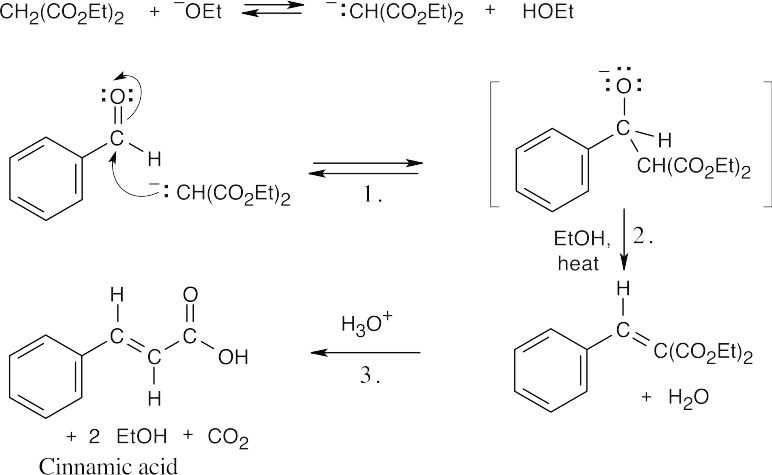
Step 1:Nucleophilic addition of the enolate to the carbonyl group.
Step 2:Protonation, dehydration.
Step 3:Ester cleavage and decarboxylation of a β-keto ester.
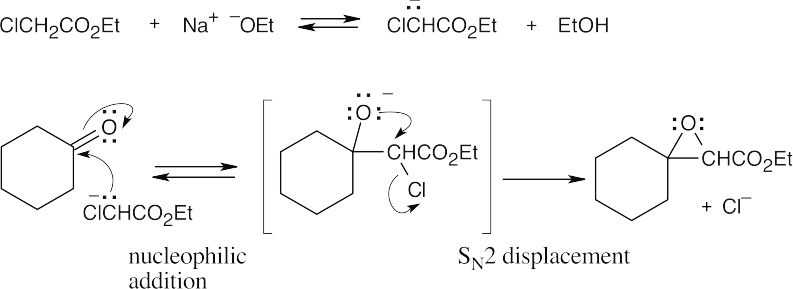
- This mechanism divides into two sequences of steps. In the first part of the mechanism, an acetal is hydrolyzed to acetone and a dihydroxycarboxylic acid.
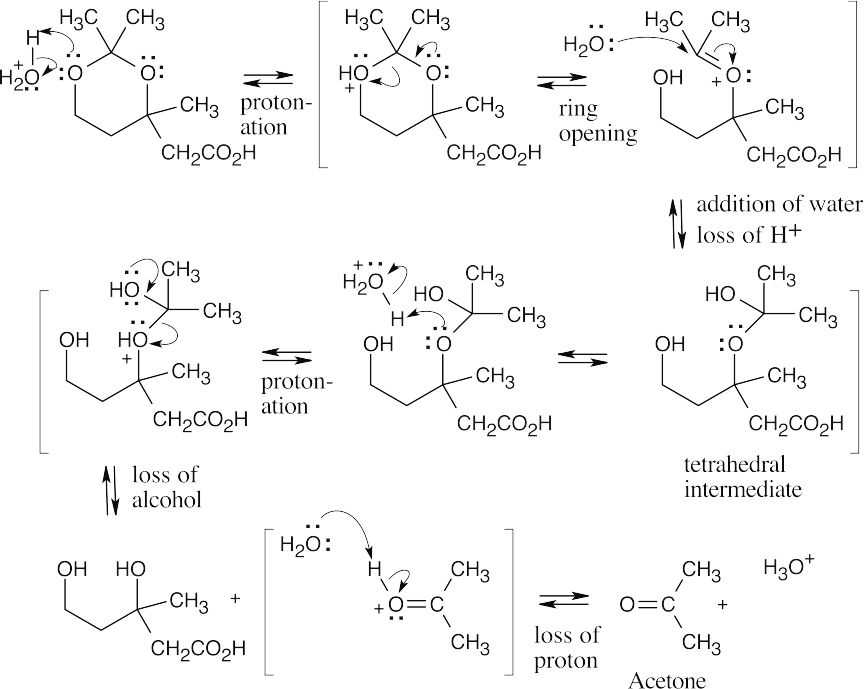
In the second set of steps, the dihydroxycarboxylic acid forms a cyclic ester (a lactone).
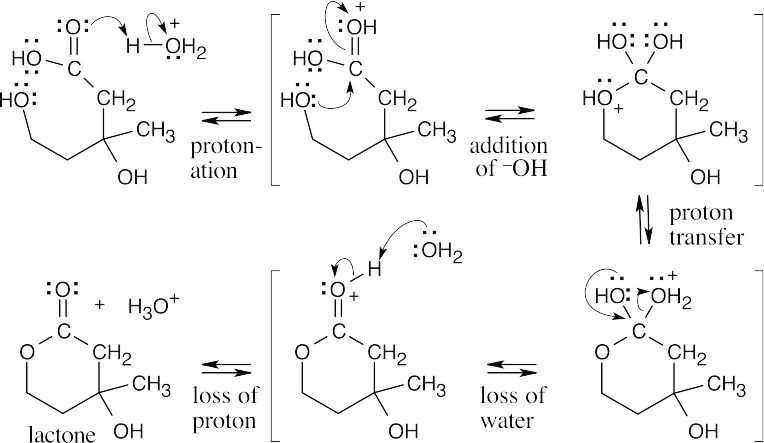
- This problem becomes easier if you draw the starting material so that it resembles the product.
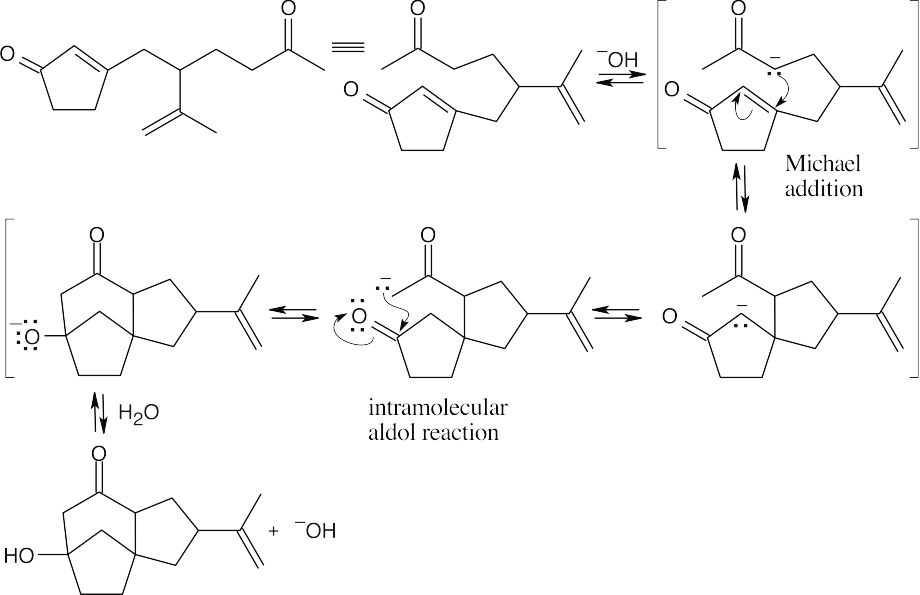
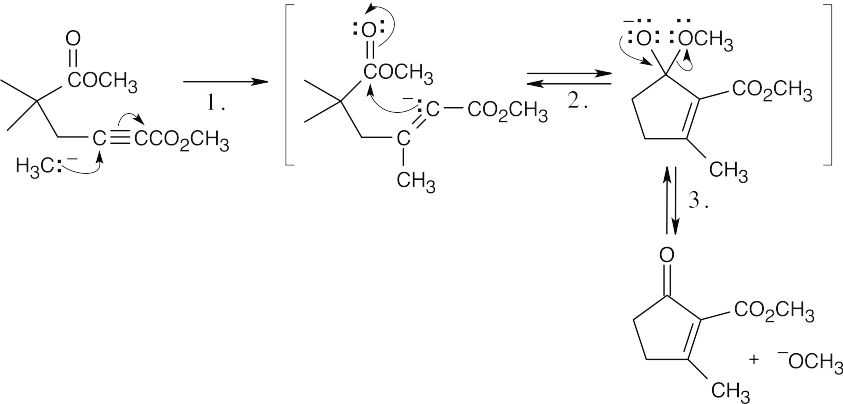
Step 1:Conjugate addition of H3C–.
Step 2:Claisen condensation.
Step 3:Loss of methoxide.
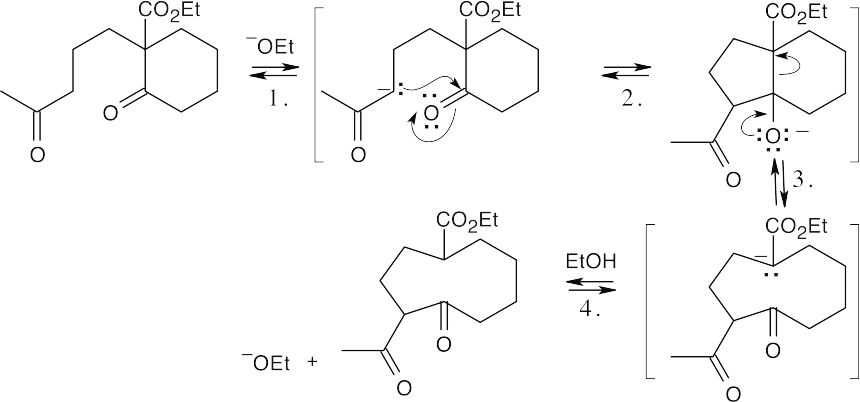
Step 1:Enolate formation.
Step 2:Intramolecular aldol condensation.
Step 3:Retro-aldol condensation.
Step 4:Protonation.
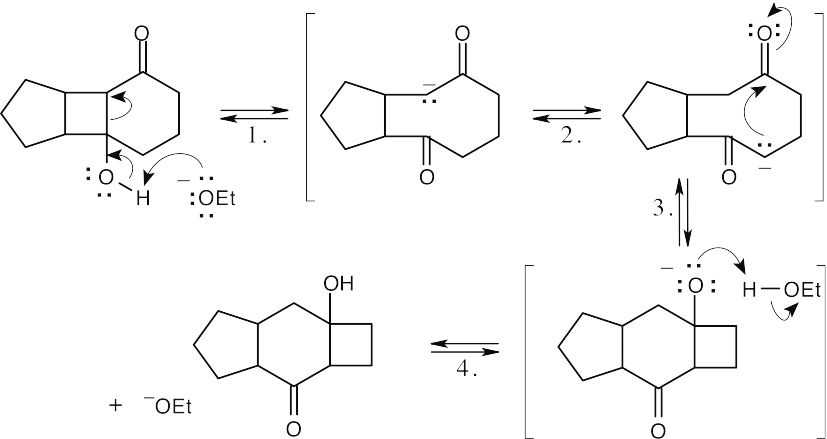
Step 1:Deprotonation and retro aldol reaction.
Step 2:Equilibration between two enolates.
Step 3:Internal aldol condensation.
Step 4:Protonation.
23.45 (a)
(b)
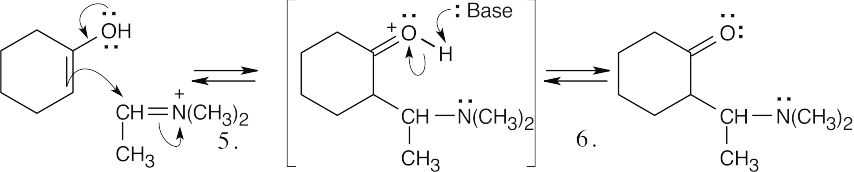
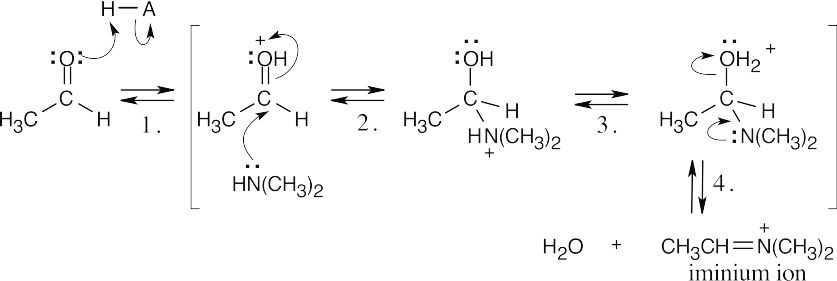
Step 1:Protonation.
Step 2:Addition of amine.
Step 3:Proton transfer.
Step 4:Elimination of water.
Step 5:Aldol-like addition of enol to iminium ion.
Step 6:Loss of proton.
23.46 The Mannich reaction occurs between the diester, butanedial, and methylamine.
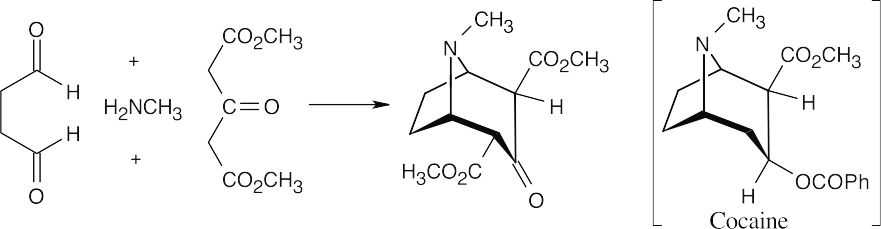
23.47
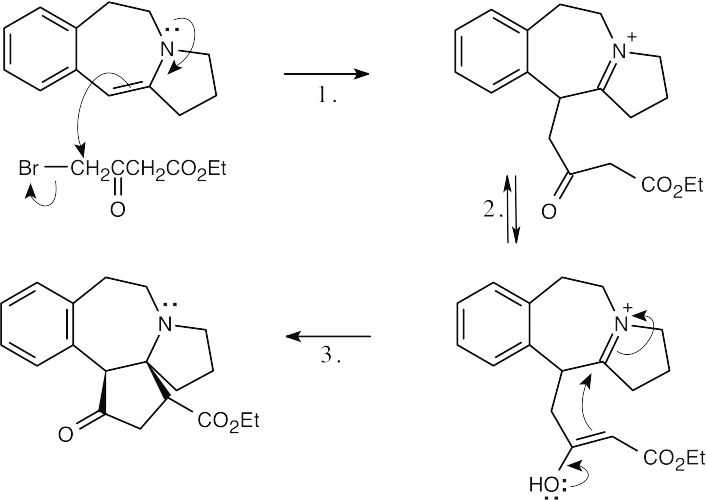
Step 1:SN2 displacement of Br– by enamine.
Step 2:Tautomerization of ketoester.
Step 3:Attack of enol on iminium ion.
Aldol Reactions
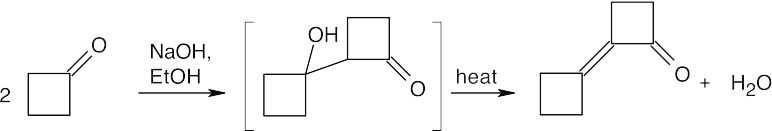 (a)(CH3)3CCHO has no α hydrogens and does not undergo aldol self-condensation. (b)
(a)(CH3)3CCHO has no α hydrogens and does not undergo aldol self-condensation. (b)
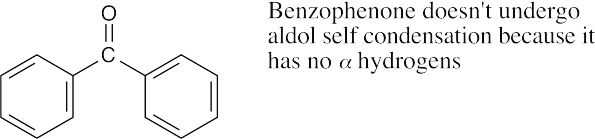 (c)
(c)
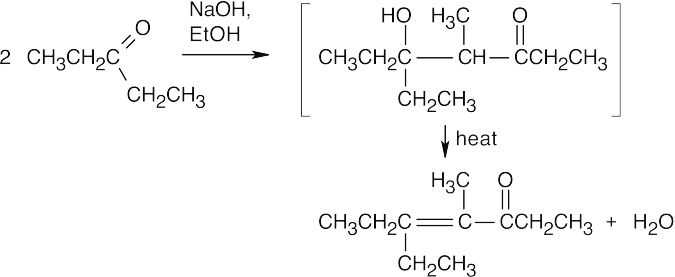 (d)
(d)
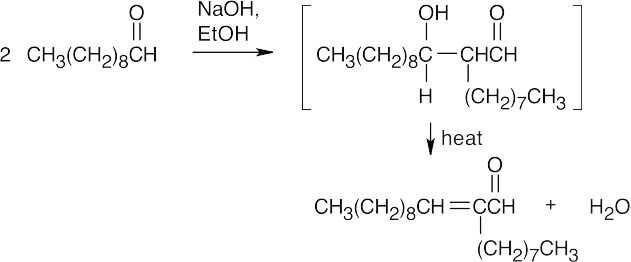 (e)
(e)
- C6H5CH=CHCHO does not undergo aldol reactions because its α proton isn’t
acidic.
- As always, analyze the product for the carbon–carbon double bond that is formed by dehydration of the initial aldol adduct. Break the bond, and add a carbonyl oxygen to the appropriate carbon to identify the carbonyl reactant(s).
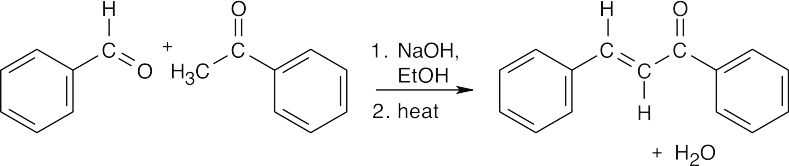 (a)
(a)
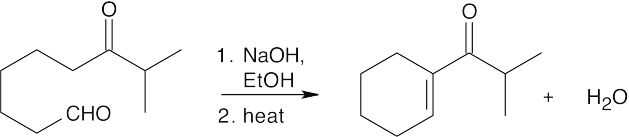 (b)
(b)
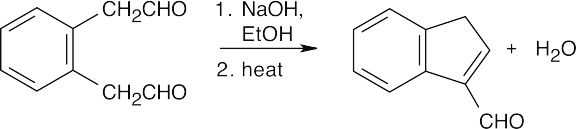 (c)
(c)
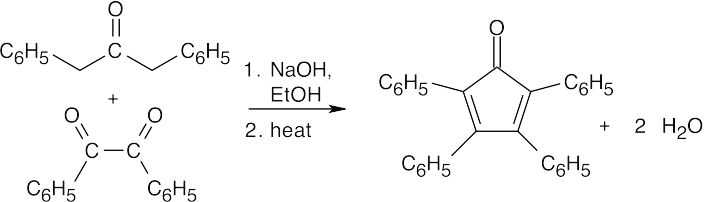 (d)
(d)
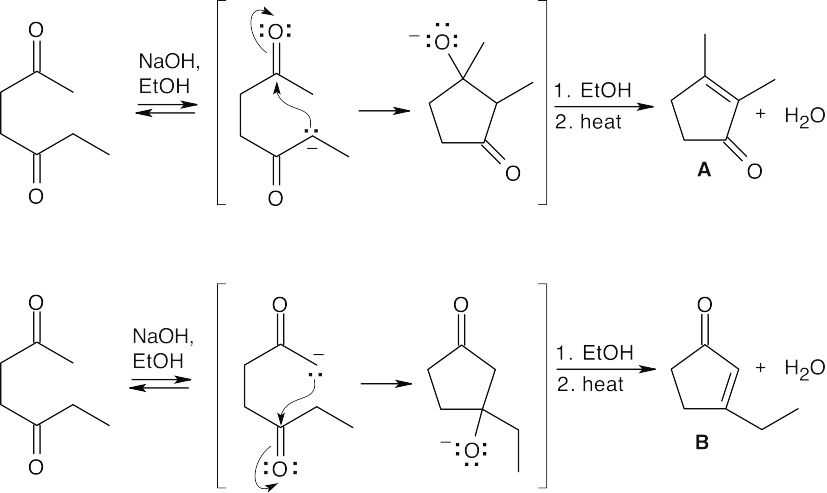
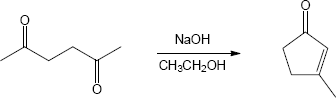
- Product A, which has two singlet methyl groups and no vinylic protons in its 1H NMR, is the major product of the intramolecular cyclization of 2,5-heptanedione.
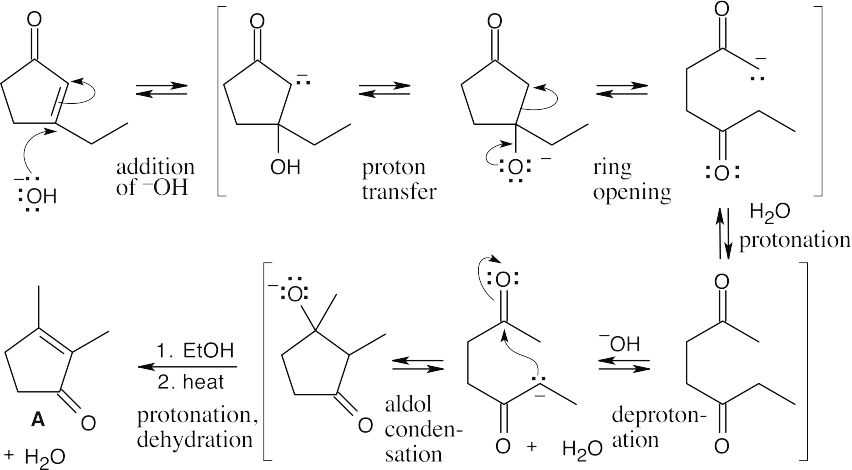
Because all steps in the aldol reaction are reversible, the more stable product is formed at equilibrium.
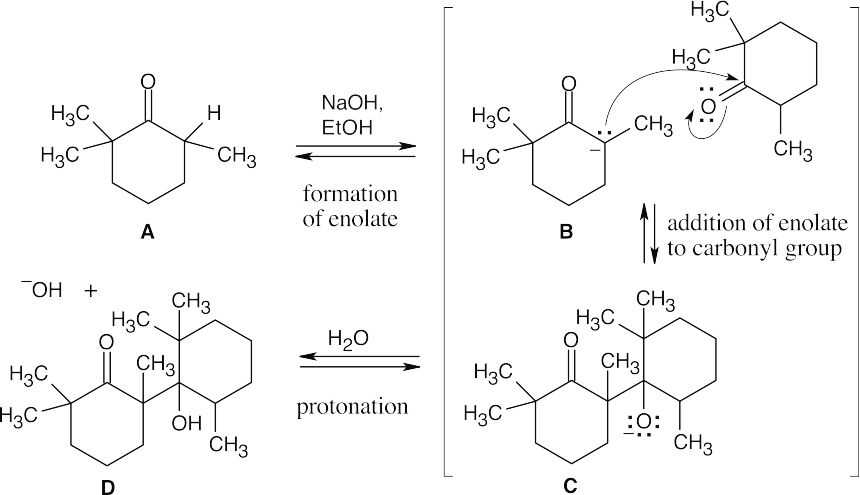
An aldol condensation involves a series of reversible equilibrium steps. In general, formation of product is favored by the dehydration of the β-hydroxy ketone to form a conjugated enone. Here, dehydration to form conjugated product can’t occur. In addition,
the B ⇌ C equilibrium favors B because of steric hindrance.
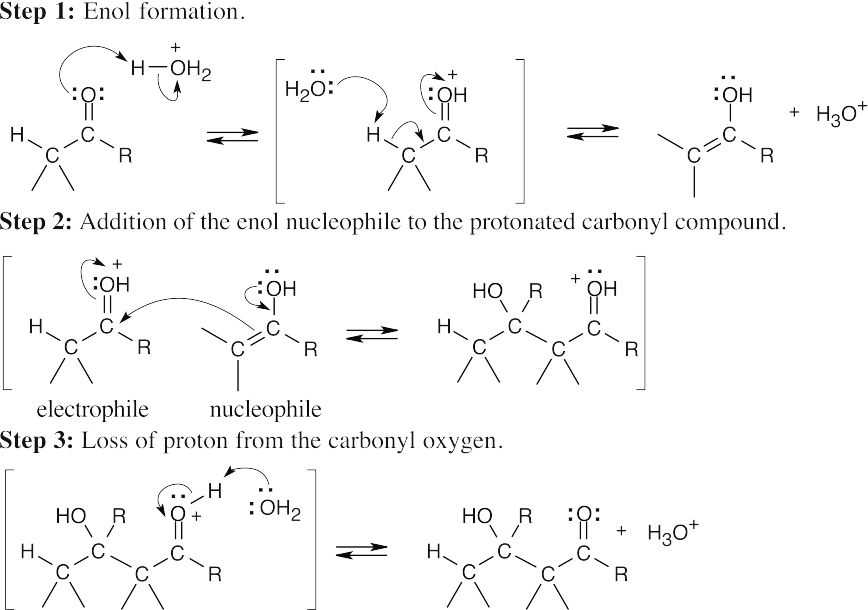
- The reactive nucleophile in the acid-catalyzed aldol condensation is the enol of one of the reactants. The electrophile is a reactant with a protonated carbonyl group.
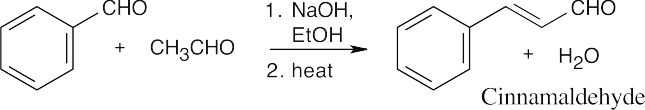
Although self-condensation of acetaldehyde can take place, the mixed aldol product predominates.
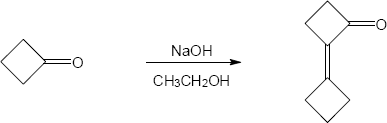
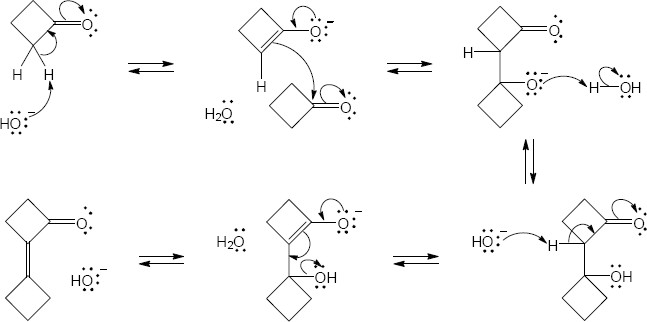
- The first step of an aldol condensation is enolate formation. The ketone shown here does not enolize because double bonds at the bridgehead of small bicyclic ring systems are too strained to form. Since the bicyclic ketone does not enolize, it doesn’t undergo aldol condensation.
Claisen Condensations
23.58 (a)
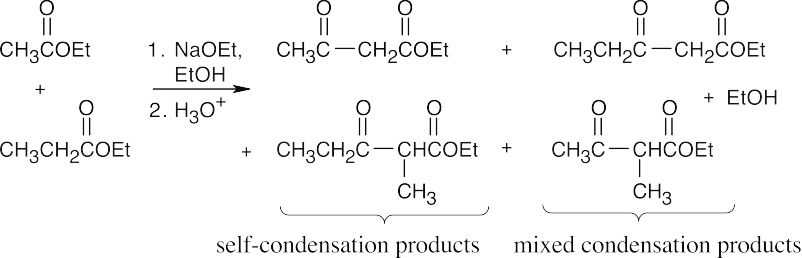
Approximately equal amounts of each product will form if the two esters are of similar reactivity.
(b)
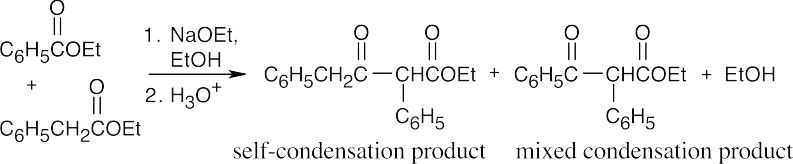
The mixed condensation product predominates.
(c)
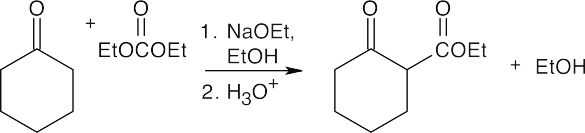
This is the only Claisen monocondensation product (aldol self-condensation of cyclohexanone also occurs to a small extent).
(d)
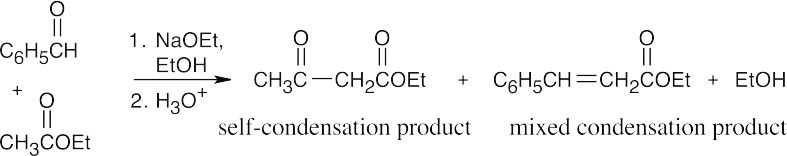
The mixed Claisen product is the major product.
23.59 If cyclopentanone and base are mixed first, aldol self-condensation of cyclopentanone can occur before ethyl formate is added. If both carbonyl components are mixed together before adding base, the more favorable mixed Claisen condensation occurs with less competition from the aldol self-condensation reaction.
23.60
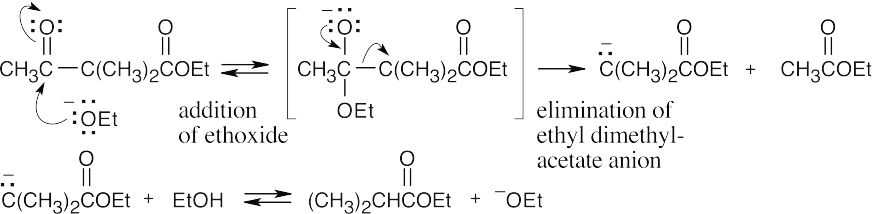
This is a reverse Claisen reaction.
- Two different reactions are possible when ethyl acetoacetate reacts with ethoxide anion. In one reaction, attack of ethoxide ion on the carbonyl carbon is followed by elimination of the anion of ethyl acetate—a reverse Claisen reaction similar to the one illustrated in Problem 23.39. More likely, however, is the acid–base reaction of ethoxide ion and
a doubly activated α-hydrogen of ethyl acetoacetate.
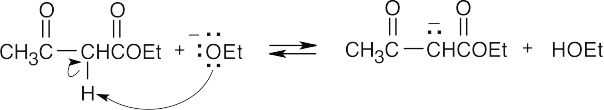
The resonance-stabilized acetoacetate anion is no longer reactive toward nucleophiles, and no further reaction occurs at room temperature. Elevated temperatures are required to make the cleavage reaction proceed. This complication doesn’t occur with ethyl dimethylacetoacetate because it has no acidic hydrogens between its two carbonyl groups.
Michael and Enamine Reactions
- Michael reactions occur between stabilized enolate anions and α,β-unsaturated carbonyl compounds. Learn to locate these components in possible Michael products. Usually, it is easier to recognize the enolate nucleophile; in (a), the nucleophile is the ethyl acetoacetate anion. The rest of the compound is the Michael acceptor. Draw a double bond in conjugation with the electron-withdrawing group in this part of the molecule.
(a)
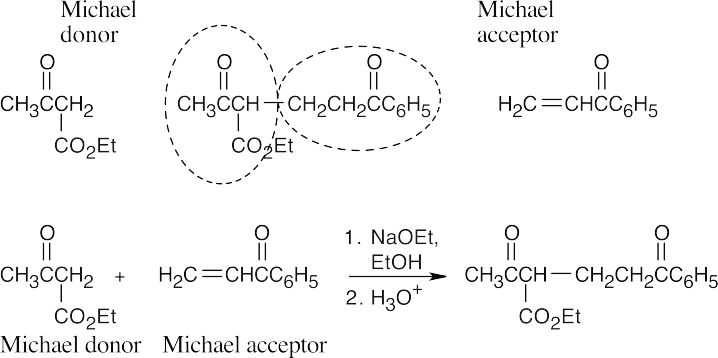
- When the Michael product has been decarboxylated after the addition reaction, it is more difficult to recognize the original enolate anion.
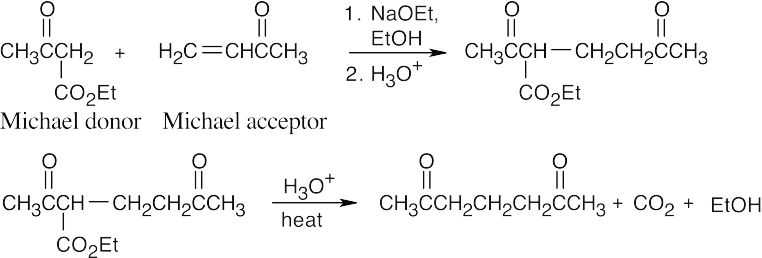
(c)
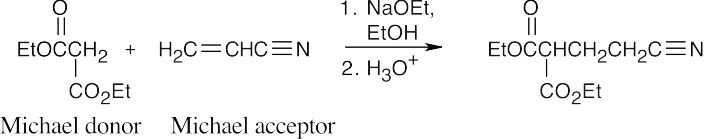
(d)
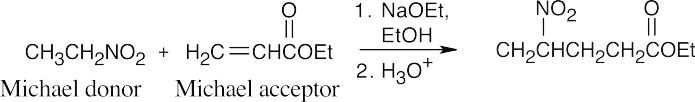
(e)
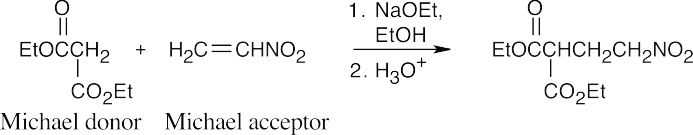
(f)
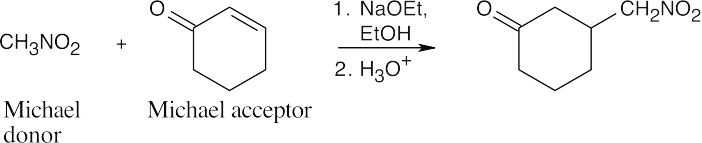
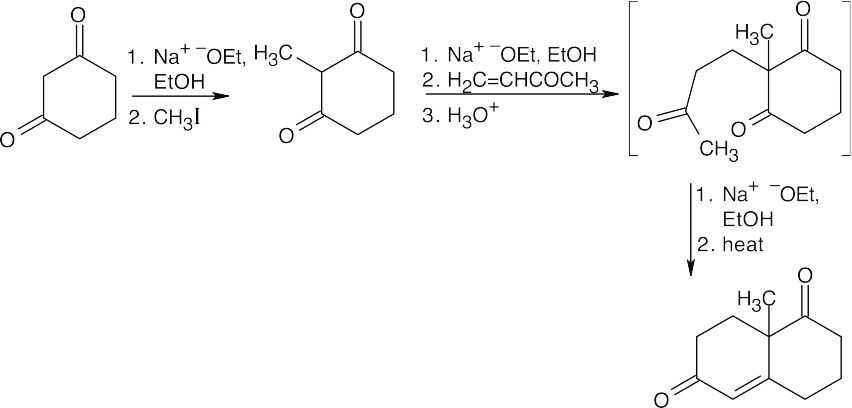
This sequence of reactions consists of an alkylation of a 1,3-diketone, followed by a Robinson annulation. The carbon–carbon double bond appears where the second carbonyl group of the diketone used to be and is the site of the ring-forming aldol reaction. A Michael reaction between the diketone and the Michael acceptor 3-buten-2- one adds the carbon atoms used to form the second ring, and an alkylation with CH3I adds the methyl group.
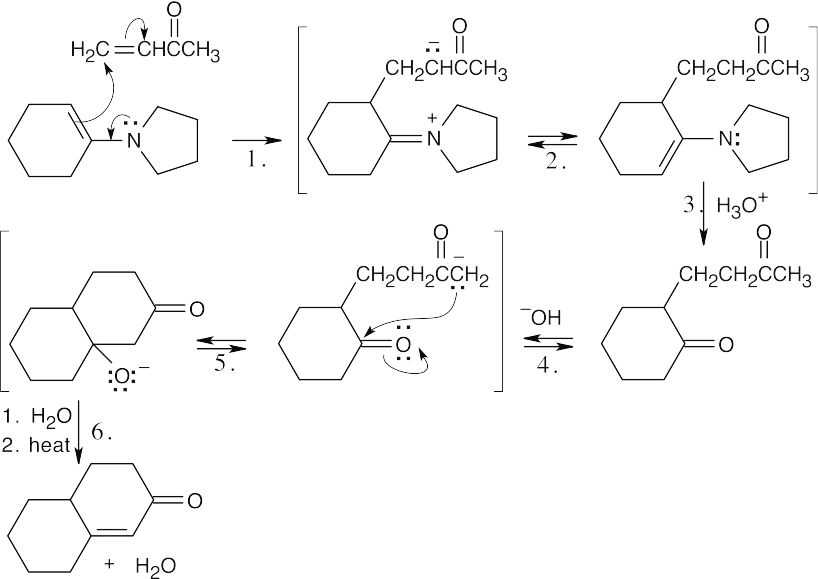
Step 1:Michael addition of enamine.
Step 2:Tautomerization.
Step 3:Enamine hydrolysis.
Step 4:Abstraction of proton by hydroxide.
Step 5:Internal aldol condensation.
Step 6:Dehydration.
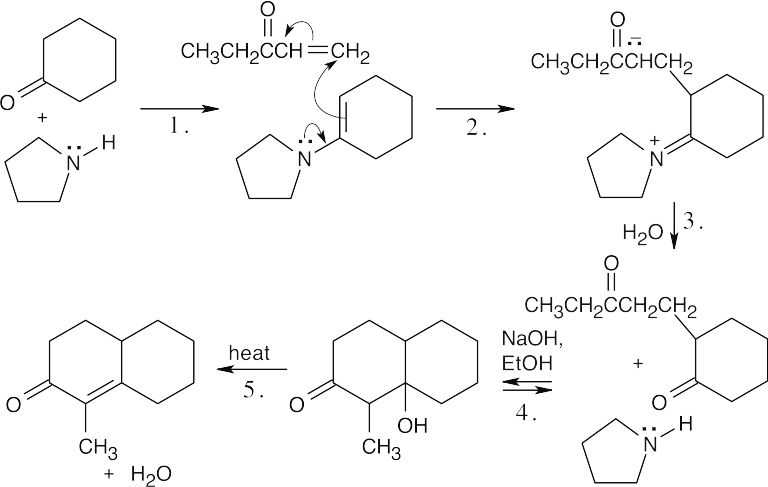 23.65 (a)
23.65 (a)
Step 1:Enamine formation.
Step 2:Michael addition of enamine.
Step 3:Enamine hydrolysis.
Step 4:Internal aldol condensation.
Step 5:Dehydration.
(b)
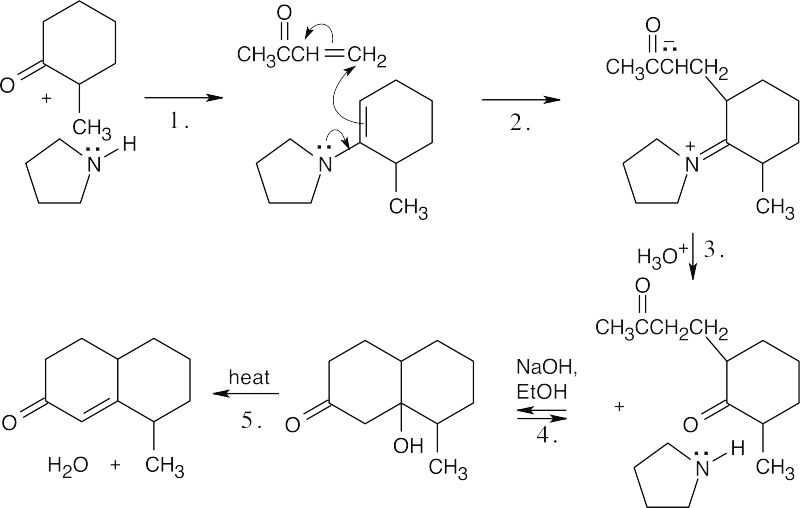
Notice that the desired enamine is formed (see Problem 23.73).
Step 1:Enamine formation.
Step 2:Michael addition of enamine.
Step 3:Enamine hydrolysis.
Step 4:Internal aldol condensation.
Step 5:Dehydration.
(c)
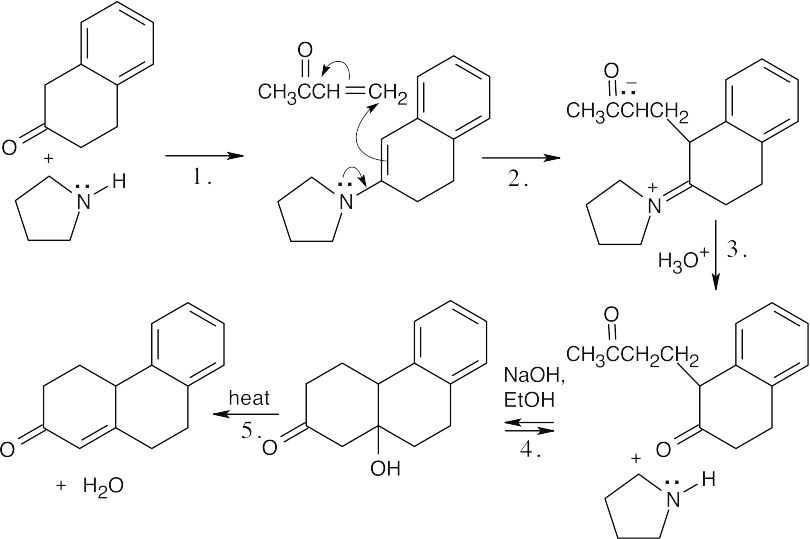
The enamine double bond is conjugated with the aromatic ring.
Step 1:Enamine formation.
Step 2:Michael addition of enamine.
Step 3:Enamine hydrolysis.
Step 4:Internal aldol condensation.
Step 5:Dehydration.
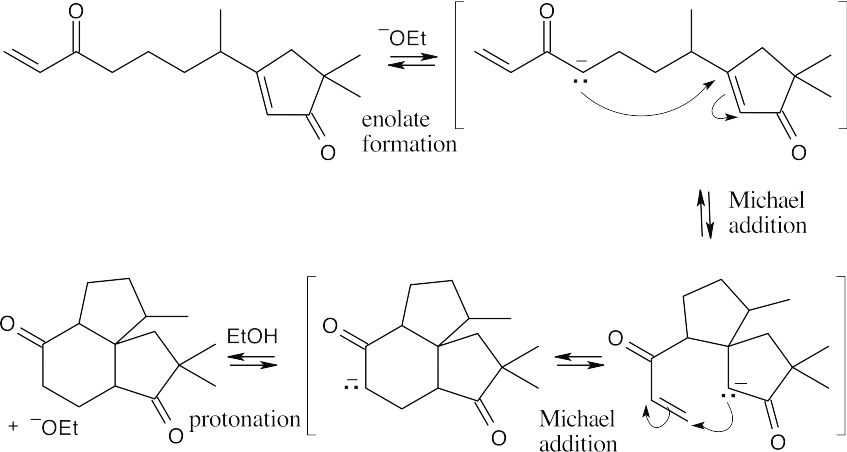
23.66
General Problems
23.67
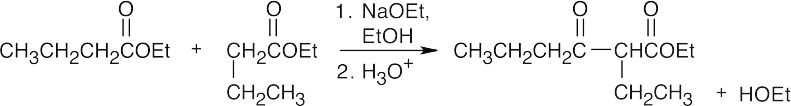 (a)
(a)
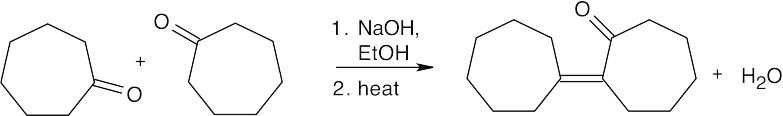 (b)
(b)
 (c)
(c)
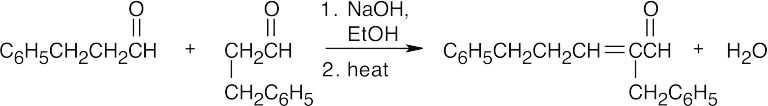 (d)
(d)
23.68 (a)Several other products are formed in addition to the one pictured. Self-condensation of acetaldehyde and acetone (less likely) can occur, and an additional mixed product is formed.
- There are two problems with this reaction. (1) Michael reactions occur in low yield with mono-ketones. Formation of the enamine, followed by the Michael reaction, gives a higher yield of product. (2) Addition can occur on either side of the ketone to give a mixture of products.
- Internal aldol condensation of 2,6-heptanedione can produce a four- membered ring or a six-membered ring. The six-membered ring is more likely to form because it is less strained.
23.69
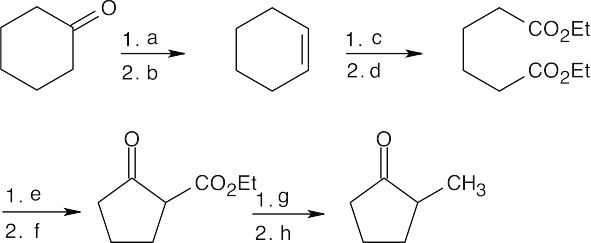
- LiAlH4, then H3O+;
- POCl3, pyridine;
- KMnO4, H3O+;
- CH3CH2OH, H+;
- Na+ –OEt;
- H3O+;
- Na+ –OEt, then CH3Br;
- H3O+, heat
23.70
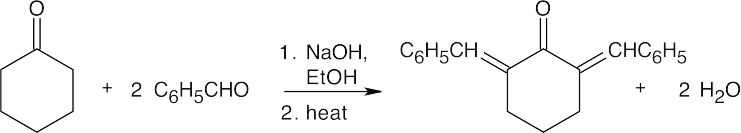 (a)
(a)
(b)
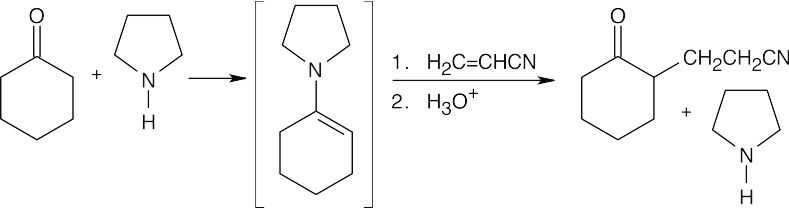
(c)
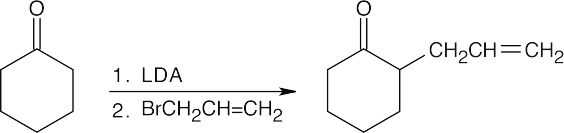
(d)
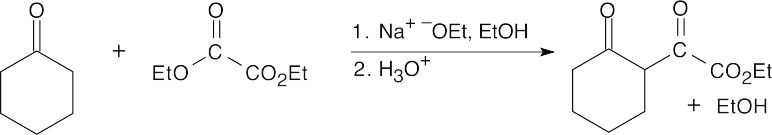
23.71 (a)
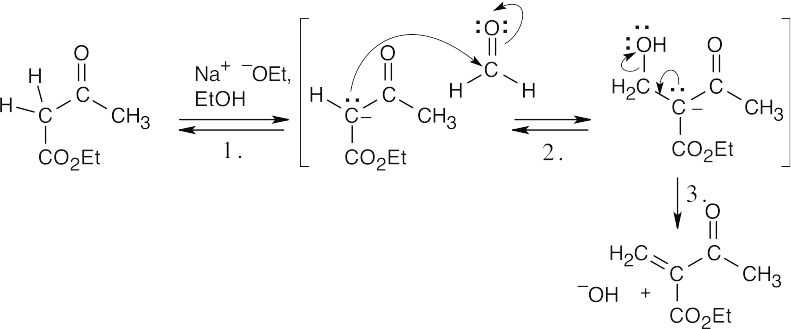
Step 1:Formation of enolate. Step 2:Aldol condensation. Step 3:Loss of hydroxide.
(b)
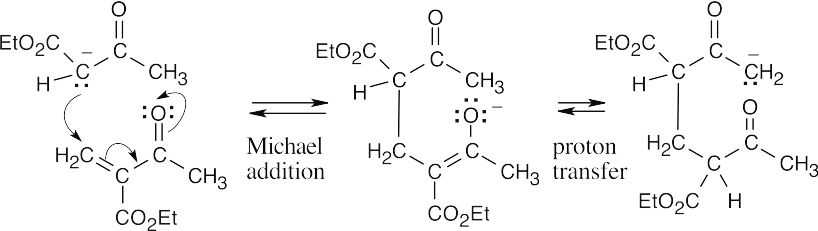
The protonated form of the above structure is the product.
23.72 (c)
(d)
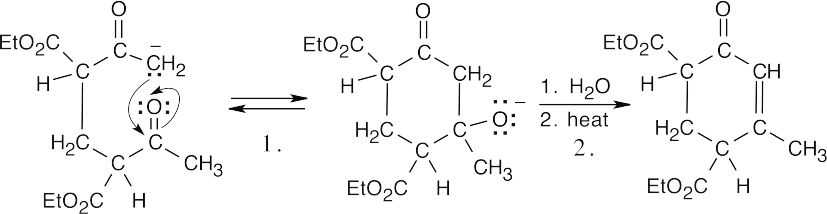
Step 1:Internal aldol condensation.
Step 2:Dehydration.
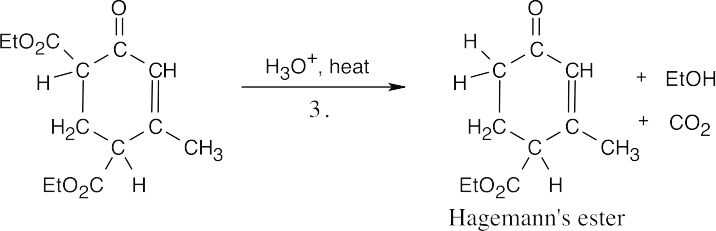
Step 3:Ester cleavage and decarboxylation of a β-keto ester.
23.73
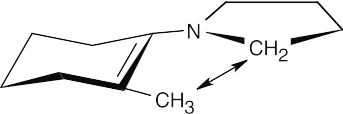
Crowding between the methyl group and the pyrrolidine ring disfavors this enamine.

The crowding in this enamine can be relieved by a ring-flip, which puts the methyl group in an axial position. This enamine is the only one formed.
This file is copyright 2023, Rice University. All Rights Reserved.

