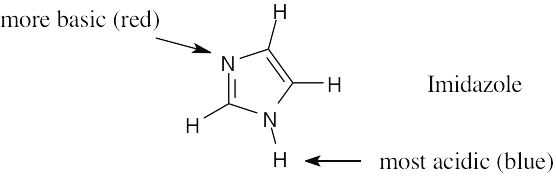
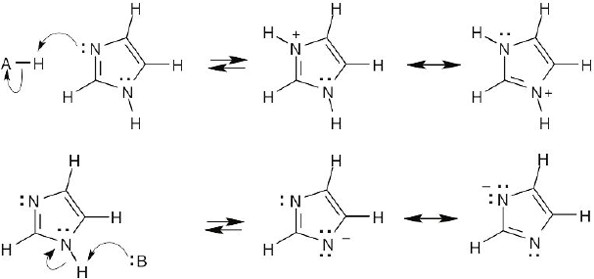
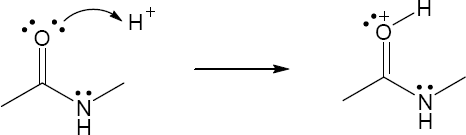
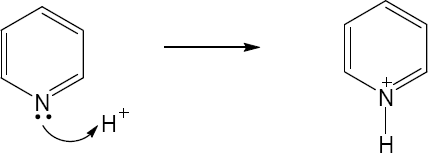
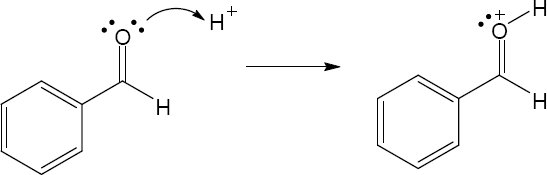
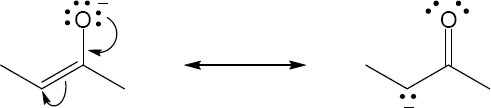
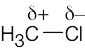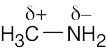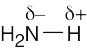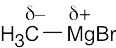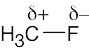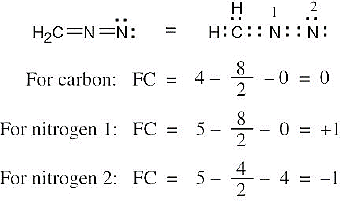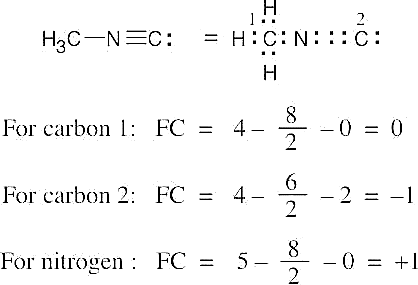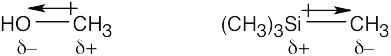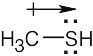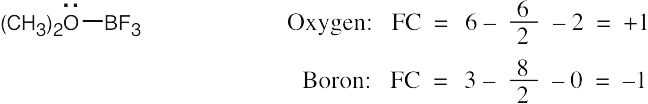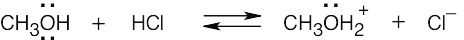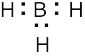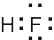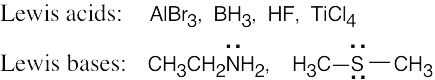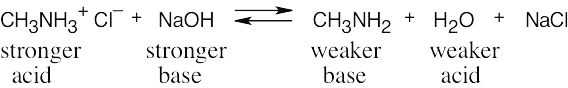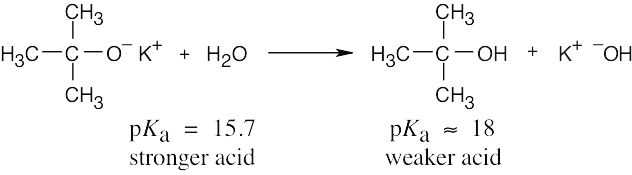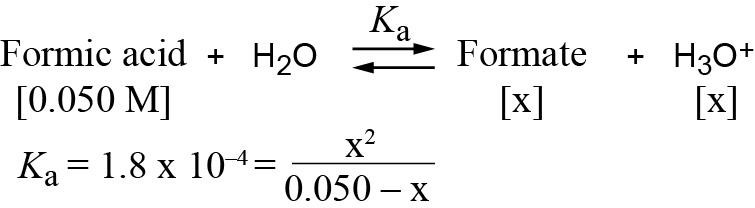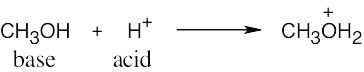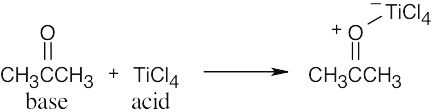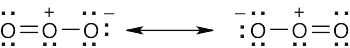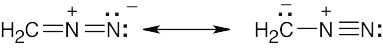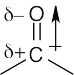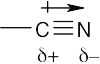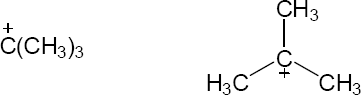2 Chapter 2 – Polar Covalent Bonds; Acids and Bases Solutions to Problems
Chapter 2 – Polar Covalent Bond; Acids and Bases
Solutions to Problems
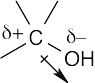
| 2.1 | After solving this problem, use Figure 2.3 to check your answers. The larger the number, the more electronegative the element. | |||||||||||||||
Carbon is slightly more electronegative than hydrogen. |
| 2.2 | As in Problem 2.1, use Figure 2.3. The partial negative charge is placed on the more electronegative atom, and the partial positive charge is placed on the less electronegative atom. | ||||||||||||
|
| 2.3 | Use Figure 2.3 to find the electronegativities of each element. Calculate ΔEN and rank the answers in order of increasing ΔEN. | ||||||||||||||||||||||||||||||||||||||||||
The most polar bond has the largest ΔEN. Thus, in order of increasing bond polarity: H3C — OH < H3C — MgBr < H3C — Li, H3C — F < H3C — K |
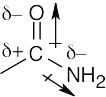
| 2.4 | In an electrostatic potential map, the color red indicates regions of a molecule that are electron-rich. The map shows that nitrogen is the most electronegative atom in chloromethane, and the direction of polarity of the C–N bond is:
|
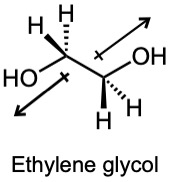
| 2.5 |
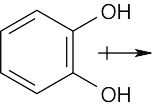
The dipole moment of ethylene glycol is zero in this conformation because the bond polarities of the two carbon–oxygen bonds cancel. However, rotation of this species about the C-C bond can result in conformers that have a non-zero dipole moment. |
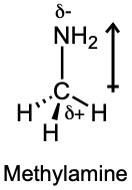
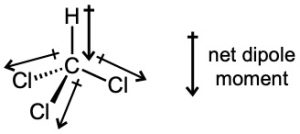
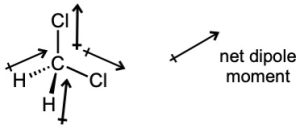
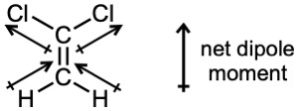
| 2.6 | For each bond, identify the more electronegative element, and draw an arrow that points from the less electronegative element to the more electronegative element. Estimate the sum of the individual dipole moments to arrive at the dipole moment for the entire molecule.
|
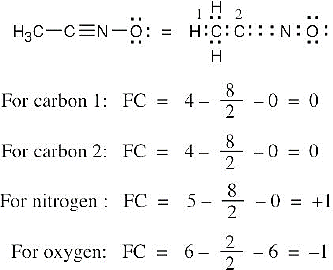
| 2.7 | To find the formal charge of an atom in a molecule, follow these two steps:
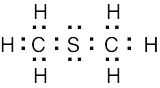
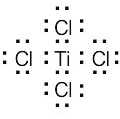
(1) Draw an electron-dot structure of the molecule. (2) Use the formula in Section 2.3 (shown below) to determine formal charge for each atom. The periodic table shows the number of valence electrons of the element, and the electron-dot structure shows the number of bonding and nonbonding electrons.
|
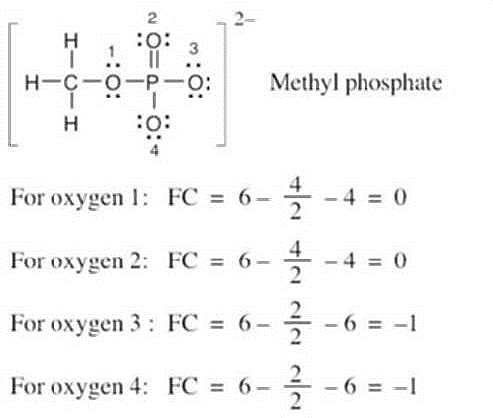
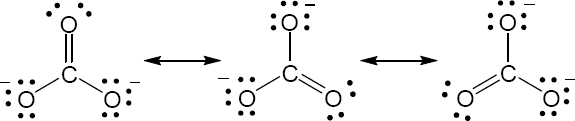
| 2.8 | 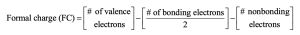
Oxygen atoms 3 and 4 each have a formal charge of –1, and oxygen atoms 1 and 2 have a formal charge of 0. |
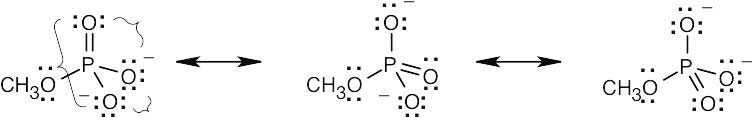
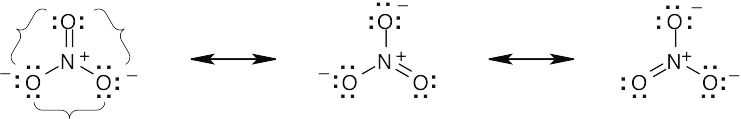
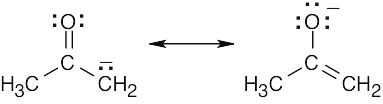
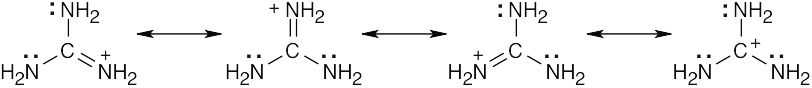
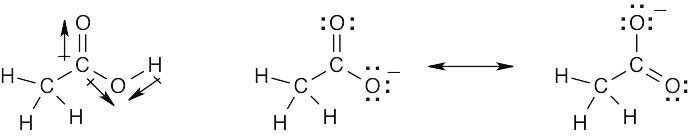
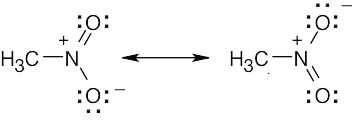
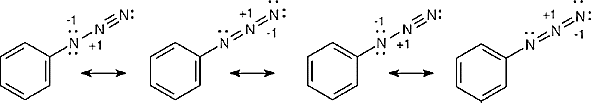
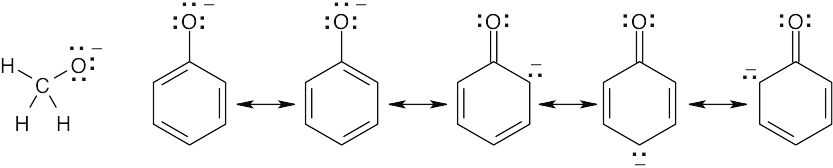
| 2.9 | Try to locate the three-atom groupings that are present in resonance forms.
|
| 2.10 | Look for three-atom groupings that contain a multiple bond next to an atom with a p orbital. Exchange the positions of the bond and the electrons in the p orbital to draw the resonance form of each grouping.
|
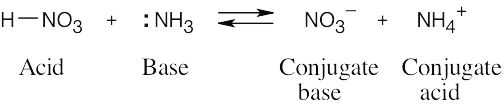
| 2.11 | When an acid loses a proton, the product is the conjugate base of the acid. When a base gains a proton, the product is the conjugate acid of the base.
|
| 2.12 | Recall from Section 2.8 that a stronger acid has a smaller pKa and a weaker acid has a larger pKa. Accordingly, phenylalanine (pKa = 1.83) is a stronger acid than tryptophan (pKa = 2.83). |
| 2.13 | HO–H is a stronger acid than H2N–H. Since H2N– is a stronger base than HO–, the conjugate acid of H2N– (H2N–H) is a weaker acid than the conjugate acid of HO– (HO–H). |
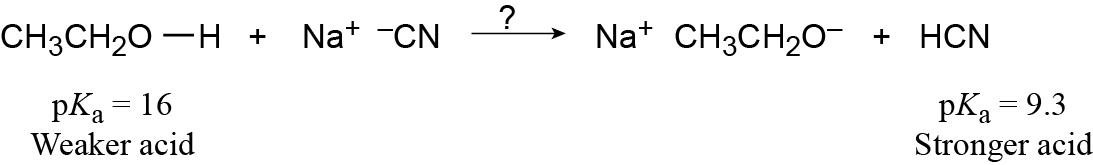
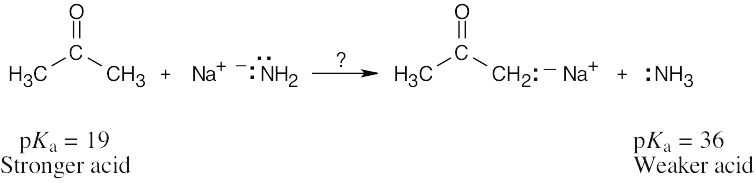
| 2.14 | Use Table 2.3 to find the strength of each acid. A reaction takes place as written if the stronger acid is the reactant.
|
| 2.15 |
As written, the above reaction will take place to virtual completion due to the large difference in pKa values. |
| 2.16 | Enter –9.31 into a calculator and use the INV LOG function to arrive at the answer: Ka = 4.9 × 10–10. |
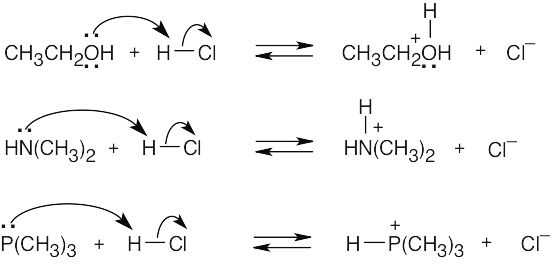
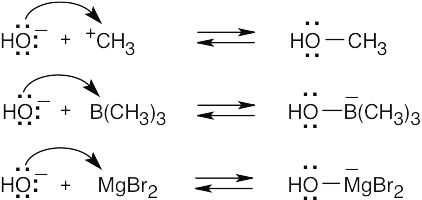
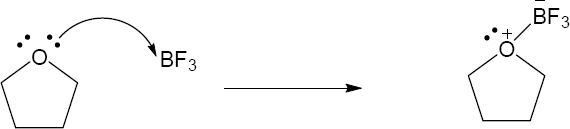
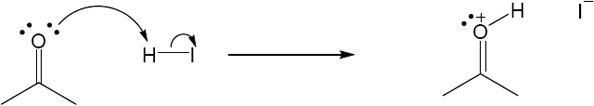
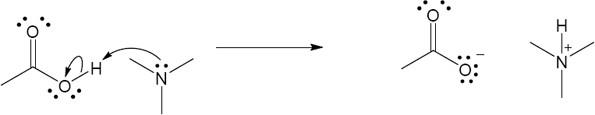
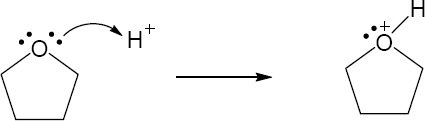
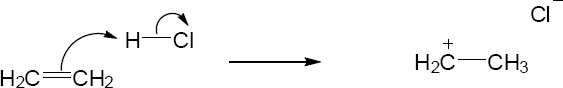
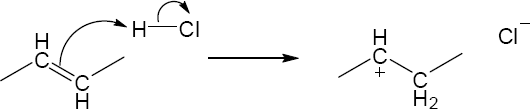
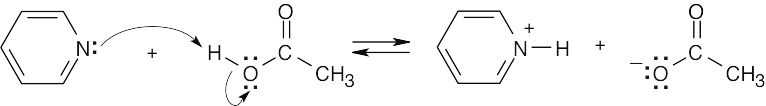
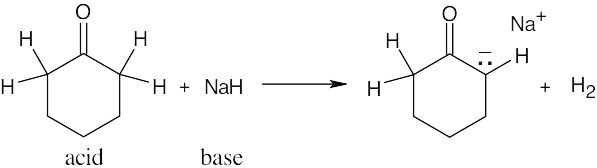
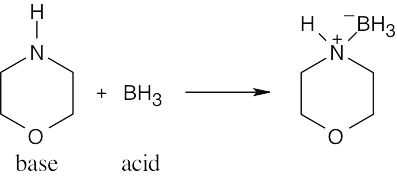
| 2.17 | Locate the electron pair(s) of the Lewis base and draw a curved arrow from the electron pair to the Lewis acid. The electron pair moves from the atom at the tail of the arrow (Lewis base) to the atom at the point of the arrow (Lewis acid). (Note: electron dots have been omitted from Cl– to reduce clutter.)
|
| 2.18 |
|
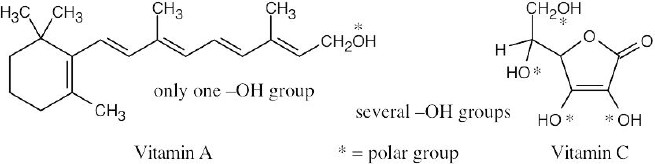
| 2.19 |
Vitamin C is water-soluble (hydrophilic) because it has several polar –OH groups that can form hydrogen bonds with water. Vitamin A is fat-soluble (hydrophobic) because most of its atoms can’t form hydrogen bonds with water. |
Additional Problems
Visualizing Chemistry
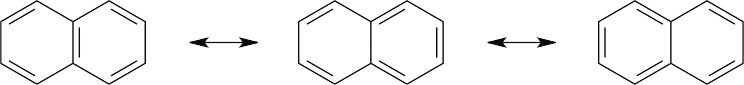
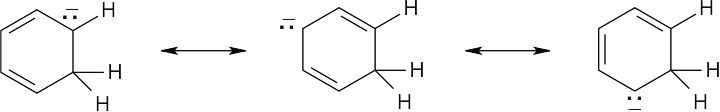
| 2.20 | Naphthalene has three resonance forms.
|
| 2.21 |
|
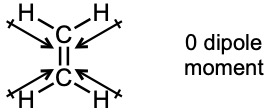
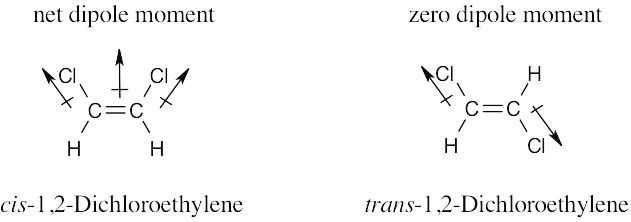
| 2.22 | Electrostatic potential maps show that the electron-rich regions of the cis isomer lie on the same side of the double bond, leading to a net dipole moment. Because the electron-rich regions of the trans isomer are symmetrical about the double bond, the individual bond dipole moments cancel, and the isomer has no overall dipole moment.
|
| 2.23 |
|
Mechanism Problems
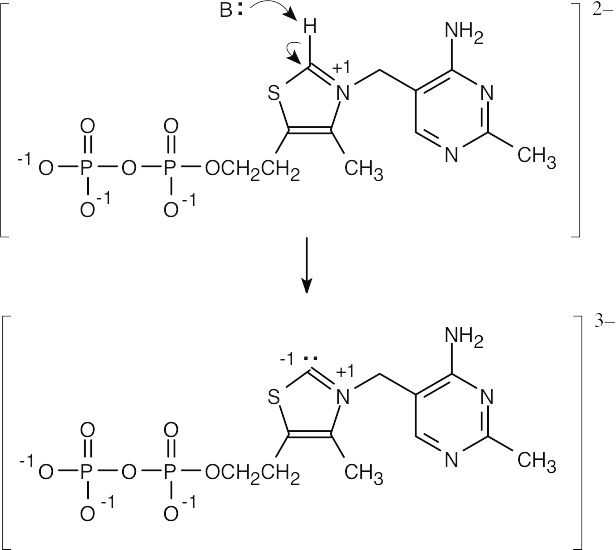
| 2.24 |
|
| 2.25 |
|
| 2.26 |
|
| 2.27 |
|
Electronegativity and Dipole Moments
| 2.28 | Use Figure 2.3 if you need help. The most electronegative element is underlined.
|
| 2.29 |
|
| 2.30 |
|
| 2.31 | (a) In Section 2.2, we found that μ = Q × r. If the two charges are separated by 136 pm, μ = 6.53 D.
(b) Since the observed dipole moment is 1.08 D, the H–Cl bond has (1.08 D / 6.53 D) × 100% = 16.5% ionic character. |
| 2.32 | In phosgene, the individual bond polarities tend to cancel, but in formaldehyde, the bond polarities add to each other. Thus, phosgene has a smaller dipole moment than formaldehyde.
|
| 2.33 | The magnitude of a dipole moment depends on both charge and distance between atoms. Fluorine is more electronegative than chlorine, but a C–F bond is shorter than a C–Cl bond. Thus, the dipole moment of CH3F is smaller than that of CH3Cl. |
| 2.34 | The observed dipole moment is due to the lone pair of electrons on sulfur.
|
Formal Charges
| 2.35 | To save space, molecules are shown as line-bond structures with lone pairs, rather than as electron-dot structures.
|
| 2.26 | As in Problem 2.31, molecules are shown as line-bond structures with lone-pair electrons indicated. Only calculations for atoms with non-zero formal charge are shown.
|
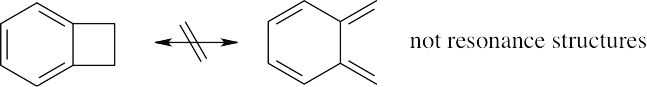
Resonance
| 2.37 | Resonance forms do not differ in the position of nuclei. The two structures in (a) are not resonance forms because the positions of the carbon and hydrogen atoms outside the ring are different in the two forms.
The pairs of structures in parts (b), (c), and (d) represent resonance forms. |
| 2.38 |
|
| 2.39 | The two structures are not resonance forms because the positions of the carbon atoms are different in the two forms. |
Acids and Bases
| 2.40 |
|
| 2.41 |
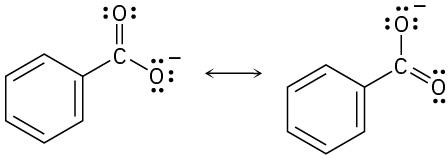
The O–H hydrogen of acetic acid is more acidic than the C–H hydrogens. The –OH oxygen is electronegative, and, consequently, the –O–H bond is more strongly polarized than the –C–H bonds. In addition, the acetate anion is stabilized by resonance. |
| 2.42
|
The Lewis acids shown below can accept an electron pair either because they have a vacant orbital or because they can donate H+. The Lewis bases have nonbonding electron pairs.
|
| 2.43 |
|
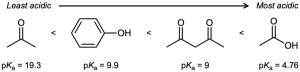
| 2.44 | The substances with the largest values of pKa are the least acidic.
|
| 2.45 | To react completely (> 99.9%) with NaOH, an acid must have a pKa at least 3 units smaller than the pKa of H2O. Thus, all substances in the previous problem except acetone react completely with NaOH. |
| 2.46 | The stronger the acid (smaller pKa), the weaker its conjugate base. Since NH4+ is a stronger acid than CH3NH3+, CH3NH2 is a stronger base than NH3. |
| 2.47 |
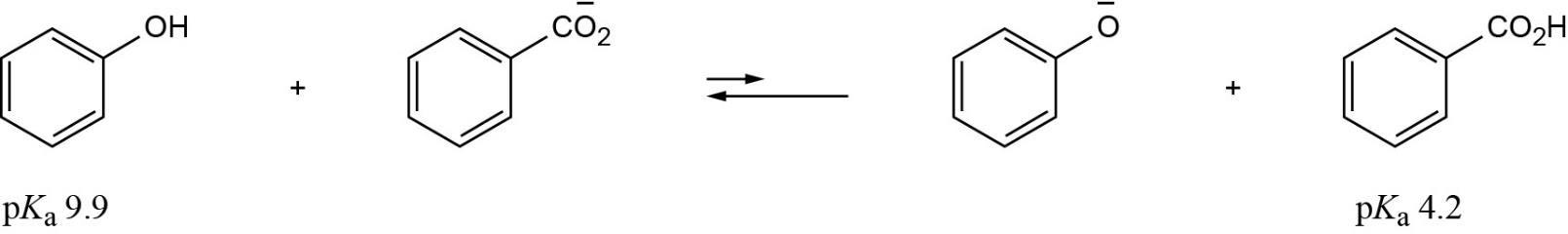
The reaction takes place as written because water is a stronger acid than tert-butyl alcohol. Thus, a solution of potassium tert-butoxide in water can’t be prepared. |
| 2.48 |
|
| 2.49 |
|
| 2.50 |
|
| 2.51 |
If you let 0.050 – x = 0.050, then x = 3.0 × 10-3 and pH = 2.52. If you calculate x exactly using the quadratic equation, then x = 2.9 × 10-3 and pH = 2.54. |
| 2.52 | Only acetic acid will react with sodium bicarbonate. Acetic acid is the only substance in Problem 2.40 that is a stronger acid than carbonic acid. |
General Problems
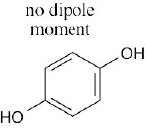
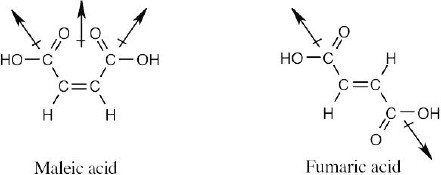
| 2.53 | In maleic acid, the individual dipole moments add to produce a net dipole moment for the whole molecule. The individual dipole moments in fumaric acid cancel, resulting in a zero dipole moment.
|
| 2.54 | Sodium bicarbonate reacts with acetic acid to produce carbonic acid, which breaks down to form CO2. Thus, bubbles of CO2 indicate the presence of an acid stronger than carbonic acid, in this case acetic acid, as the pKa values indicate. Phenol does not react with sodium bicarbonate. |
| 2.55 | Reactions (a) and (c) are reactions between Brønsted–Lowry acids and bases; the stronger acid and stronger base are identified. Reactions (b) and (d) occur between Lewis acids and bases.
|
| 2.56 | Pairs (a) and (d) represent resonance structures; pairs (b) and (c) do not. For two structures to be resonance forms, all atoms must be in the same positions in all resonance forms. |
| 2.57 |
|
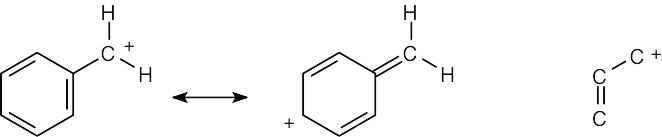
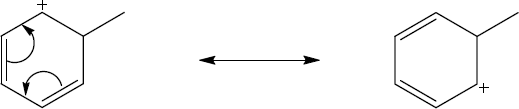
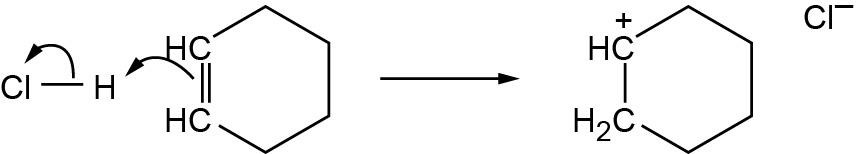
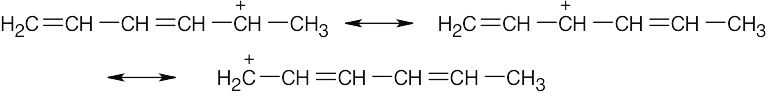
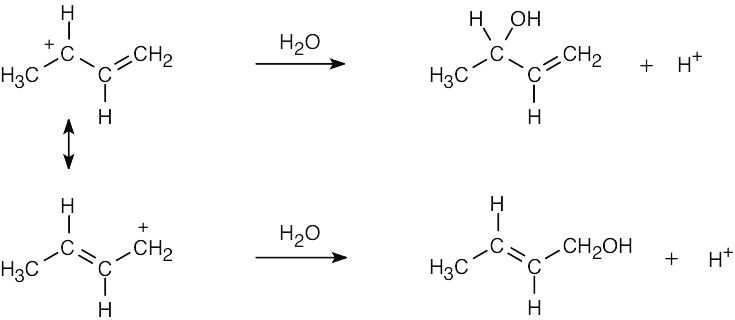
| 2.58 | The cation can be represented by two resonance forms. Reaction with water can occur at either positively charged carbon, resulting in two products.
|
| 2.59 |
|
| 2.60 |
|
| 2.61 |
When phenol loses a proton, the resulting anion is stabilized by resonance. The methanol anion is not stabilized by resonance. |
| 2.62 |
|
| 2.63 |
|
| 2.64 | The equilibrium always favors the weaker acid/base pair (acid with the higher pKa).
|
| 2.65 |
|