27 Chapter 27 – Biomolecules: Lipids Solutions to Problems
27.1
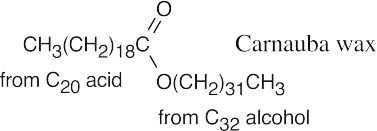
27.2
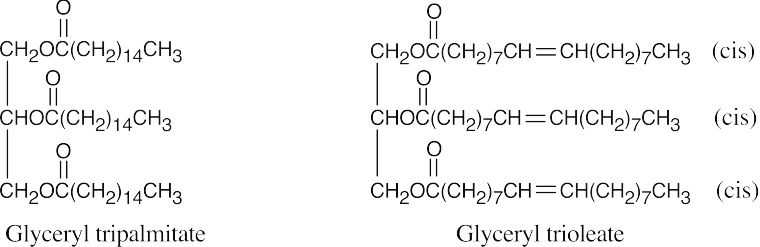
Glyceryl tripalmitate is higher melting because it is saturated.
27.3
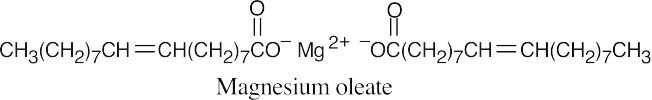
The double bonds are cis.
27.4
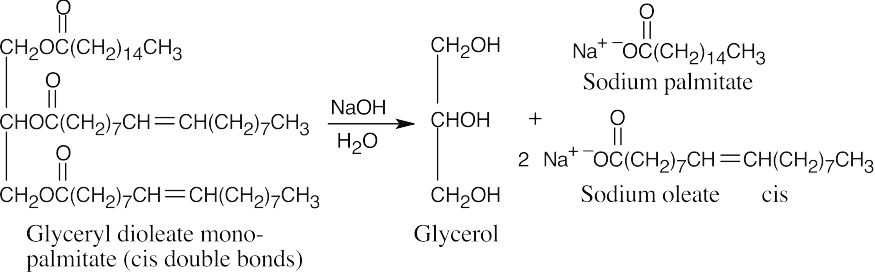
27.5
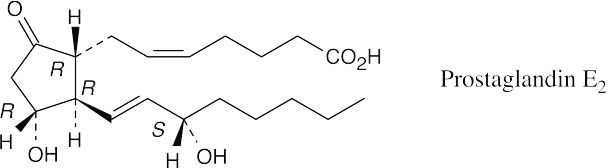
The pro-S hydrogen (blue) ends up cis to the methyl group, and the pro-R hydrogen (red) ends up trans.
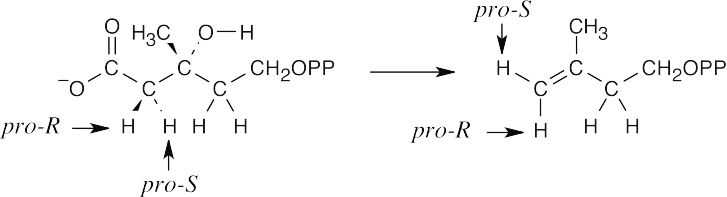
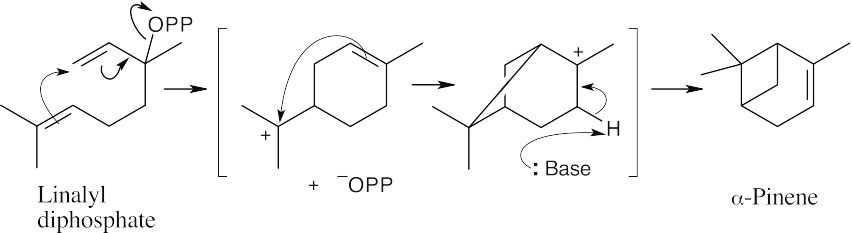
As described in Worked Example 27.1, draw the diphosphate precursor so that it resembles the product. Often, the precursor is linalyl diphosphate, which results from isomerization of geranyl diphosphate (the mechanism is shown in Figure 27.11). In (a), it’s not easy to see the relationship, but once you’ve arrived at the product, rotate the structure.
(a)
(b)
Both ring systems are trans-fused, and both hydrogens at the ring junctions are axial. Refer back to Chapter 4 if you have trouble remembering the relationships of substituents on a cyclohexane ring.
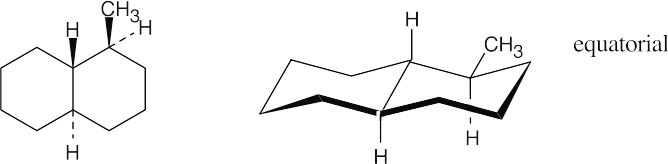 (a)
(a)
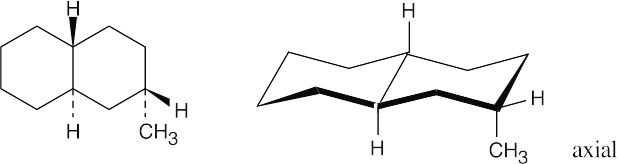 (b)
(b)
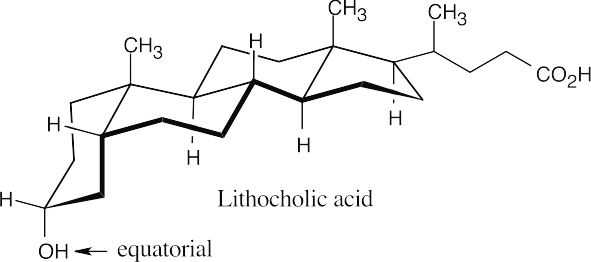
Draw the three-dimensional structure and note the relationship of the hydroxyl group to groups whose orientation is known.
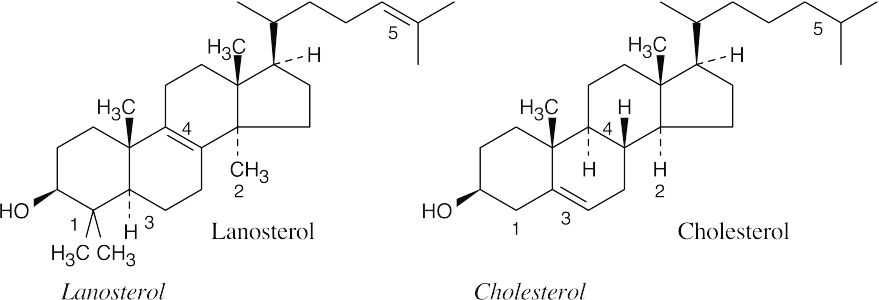
Two methyl groups at C4.1.Two hydrogens at C4.
One methyl group at C14.2.One hydrogen at C14.
C5–C6 single bond.3.C5–C6 double bond.
C8–C9 double bond.4.C8–C9 single bond.
Double bond in side chain5.Saturated side chain.
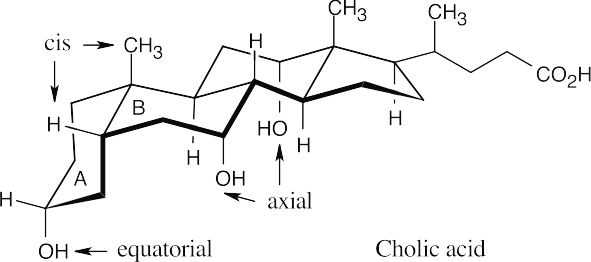 Additional Problems Visualizing Chemistry 27.11
Additional Problems Visualizing Chemistry 27.11
Cholic acid is an A–B cis steroid because the groups at the fusion of ring A and ring B have a cis relationship.
27.12
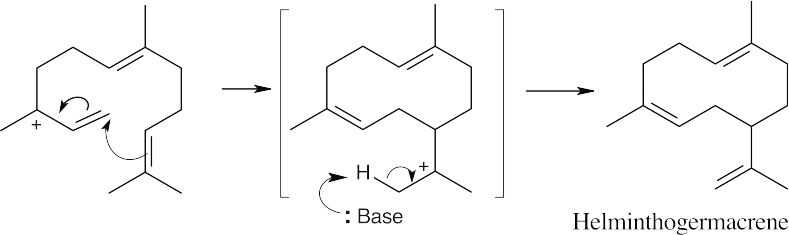
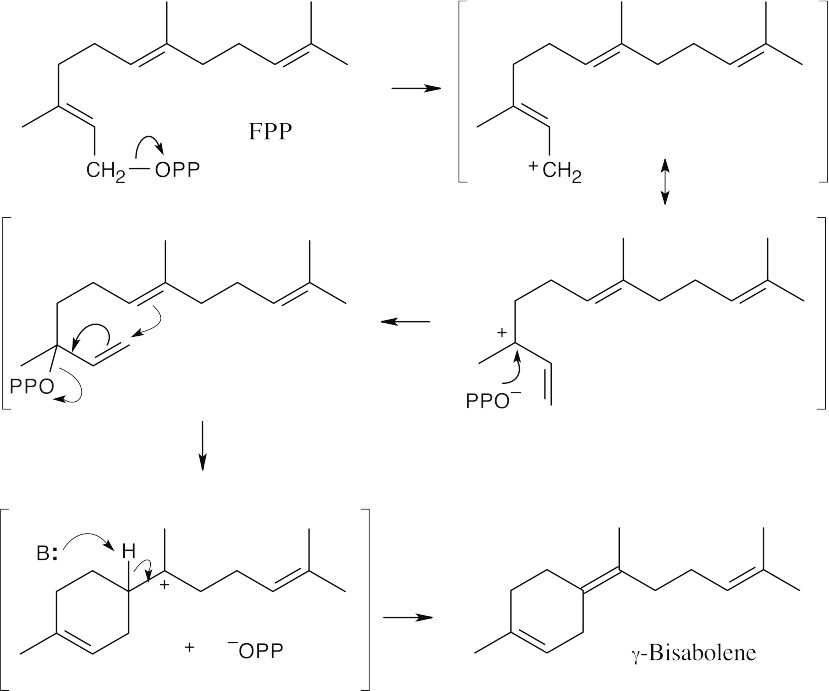
Draw farnesyl diphosphate in the configuration that resembles the product, then draw its allylic isomer (the mechanism for the formation of the isomer is shown in Problem 27.7). In this reaction, a cyclization, followed by loss of a proton to form the double bond, gives helminthogermacrene.
27.13
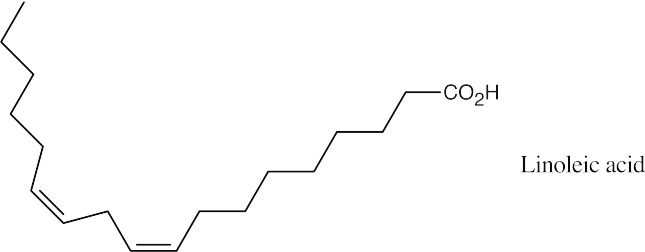
A polyunsaturated fat such as linoleic acid is more likely to be found in peanut oil.
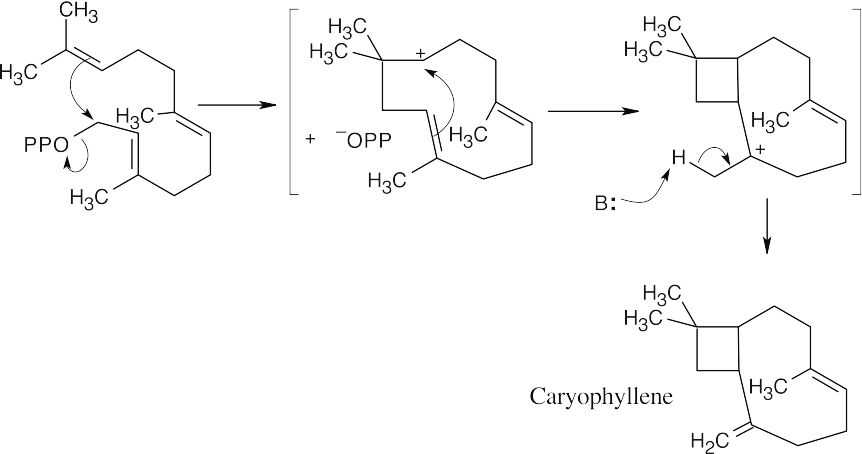 Mechanism Problems 27.14
Mechanism Problems 27.14
Draw farnesyl diphosphate in the correct orientation in order to make this problem much easier. Internal displacement of –OPP by the electrons of one double bond is followed by attack of the electrons of the second double bond on the resulting carbocation. Loss of a proton from the carbon next to the resulting carbocation produces the double bond.
27.15
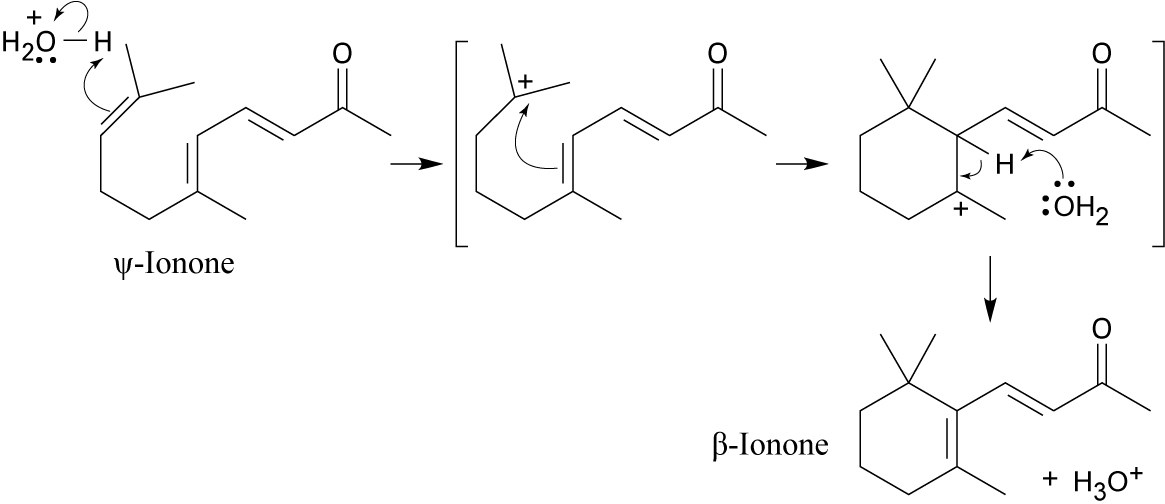
Acid protonates a double bond, and the electrons of a second double bond attack the carbocation. Deprotonation yields β-ionone.
27.16
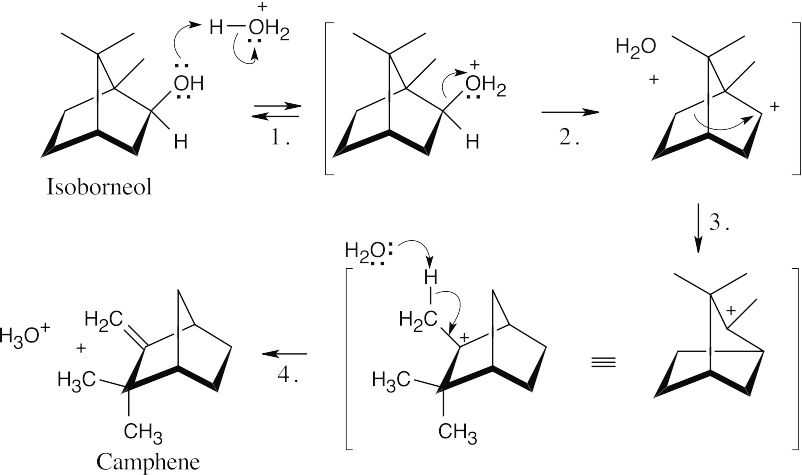
Step 1:Protonation.
Step 2:Loss of water.
Step 3:Carbocation rearrangement.
Step 4:Loss of proton.
The key step is the carbocation rearrangement, which occurs by the migration of one of the ring bonds.
 Fats, Oils, and Related Lipids 27.17
Fats, Oils, and Related Lipids 27.17
27.18
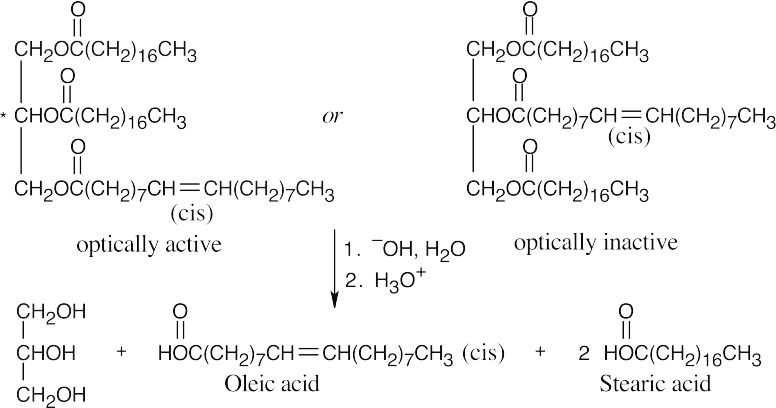
Four different groups are bonded to the central glycerol carbon atom in the optically active fat.
27.19
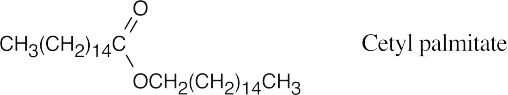
27.20
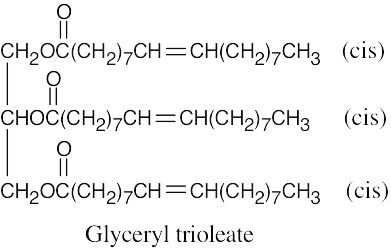
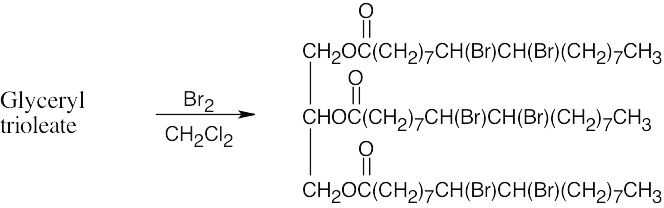 (a)
(a)
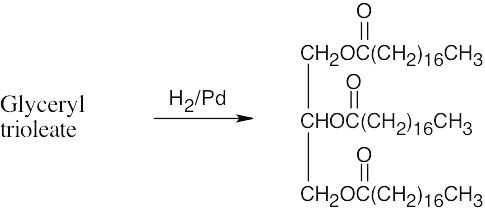 (b)
(b)
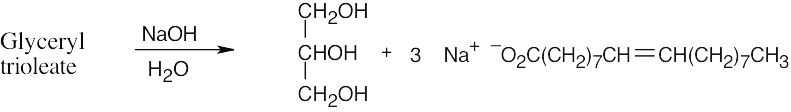 (c)
(c)
 (d)
(d)
 (e)
(e)
 (f)
(f)
27.21
7
CH3 (CH2 )
7
CH=CH (CH2 )
CO2H(cis)
Oleic Acid
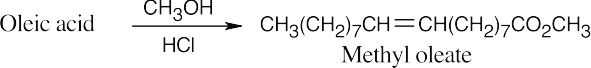 (a)
(a)
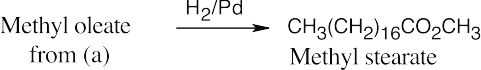 (b)
(b)
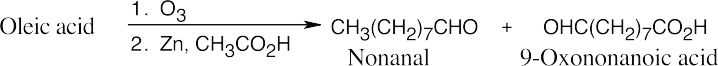 (c)
(c)
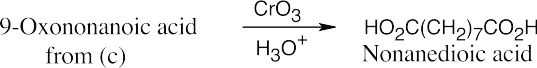 (d)
(d)
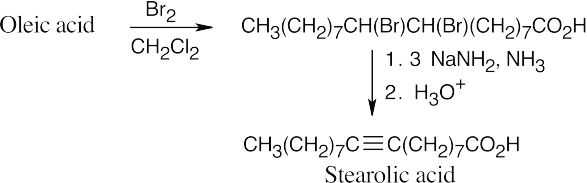 (e)
(e)
Three equivalents of the base are needed because one of them is neutralized by the carboxylic acid.
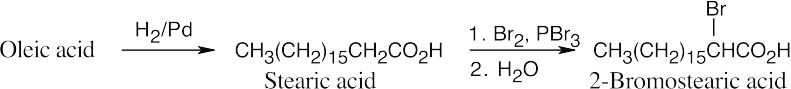 (f)
(f)
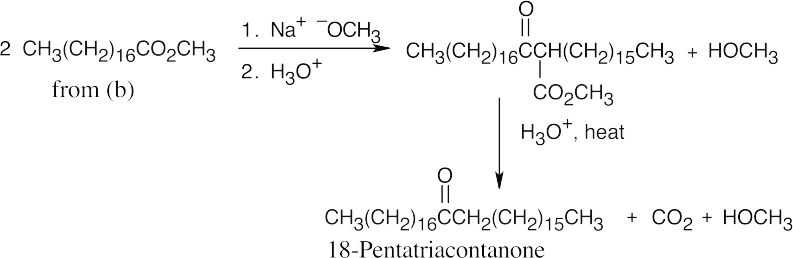 (g)
(g)
This synthesis uses a Claisen condensation, followed by a β-keto ester decarboxylation.
27.22 Fats and plasmalogens are both esters of a glycerol molecule that has carboxylic acid ester groups at C2 and C3. The third group bonded to glycerol, however, differs with the type of lipid: a fat has a carboxylic acid ester at C1, and a plasmalogen has a vinyl ether in that location.
27.23
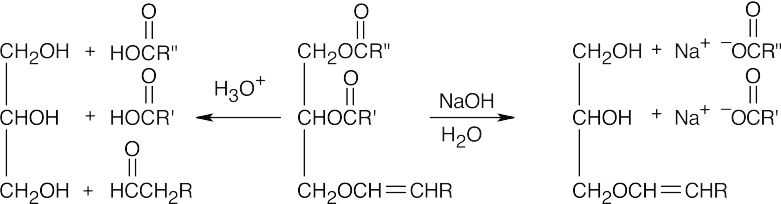
Basic hydrolysis cleaves the carboxylic acid ester bonds but doesn’t affect the ether bond. Acidic hydrolysis cleaves all three groups bonded to glycerol and produces an aldehyde from the vinyl ether group.
27.24
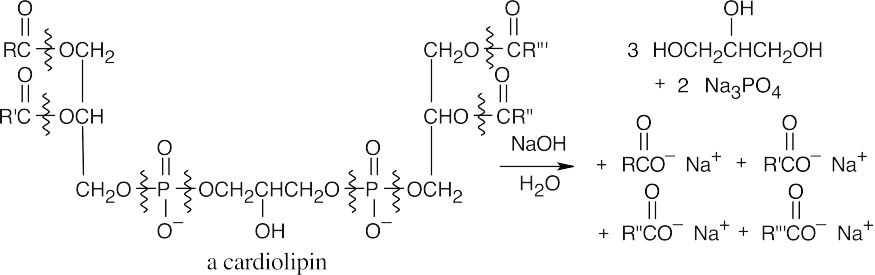
Saponification of a cardiolipin yields 4 different carboxylates, 3 equivalents of glycerol and two equivalents of phosphate.
27.25
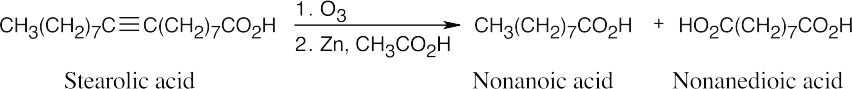
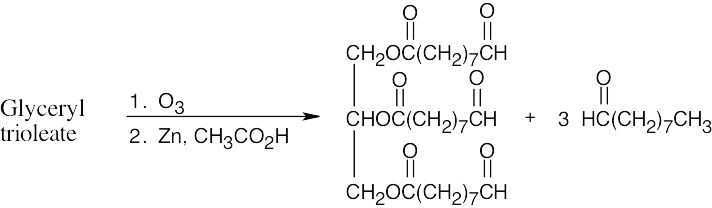
Stearolic acid contains a triple bond because the products of ozonolysis are carboxylic acids.
27.26
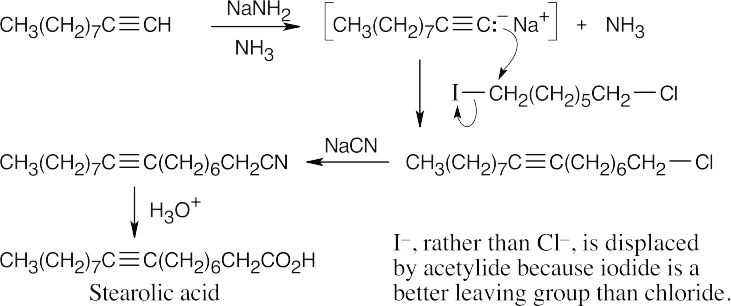
Terpenoids and Steroids
27.27–27.29
Remember that a compound with n chirality centers can have a maximum of 2n stereoisomers. Not all the possible stereoisomers of these compounds are found in nature or can be synthesized. Some stereoisomers have highly strained ring fusions; others contain 1,3-diaxial interactions.
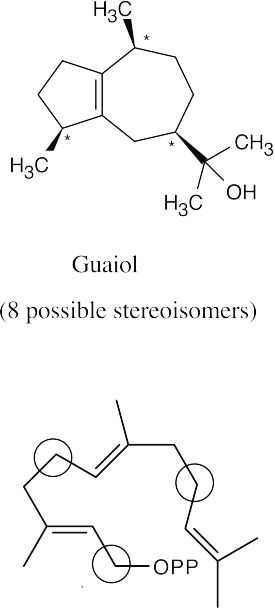
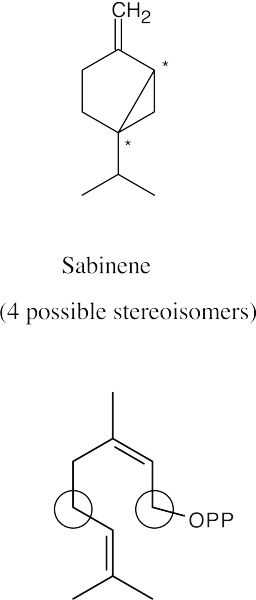 (a)(b)
(a)(b)
If carbon 1 of each diphosphate were isotopically labeled, the labels would appear at the circled positions of the terpenoids.
27.30
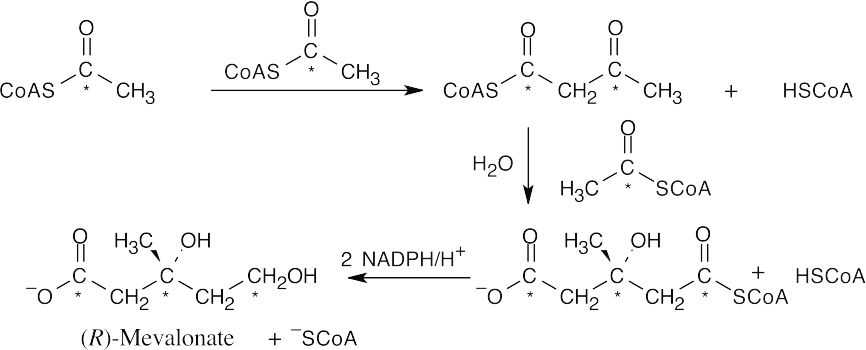
First, mevalonate 5-diphosphate is converted to isopentenyl diphosphate (IPP) and dimethallyl diphosphate (DMAPP).

IPP is isomerized to DMAPP.
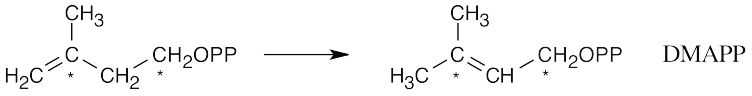
DMAPP and IPP couple to give geranyl diphosphate (GPP).
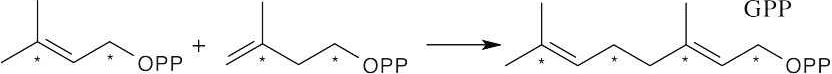
A second molecule of IPP adds to GPP to give farnesyl diphosphate, the precursor to α-cadinene.
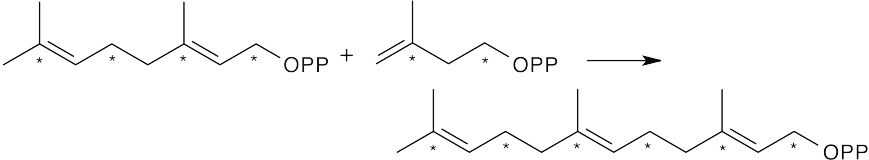
Notice that the 14C labels are located at two different positions: (1) at the carbon to which
–OPP was bonded; (2) at the carbon bonded to the methyl group.
Now, arrange farnesyl diphosphate to resemble the skeleton of α-cadinene. The first step in the reaction sequence is formation of the allylic isomer of FPP; the mechanism was shown in Problem 27.7.
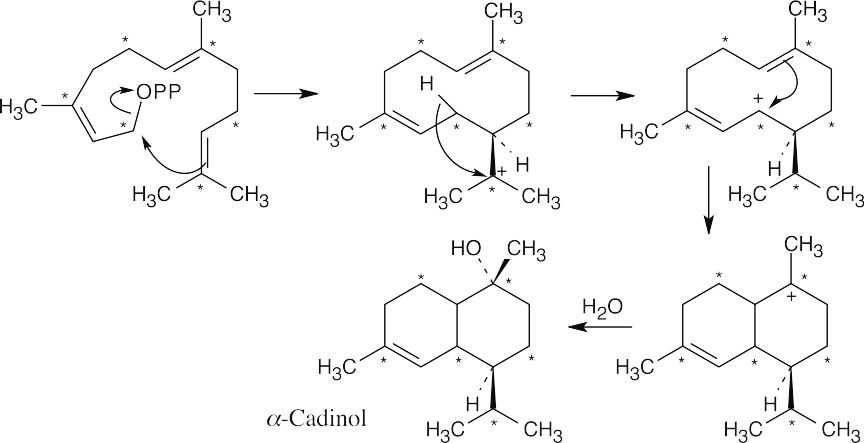
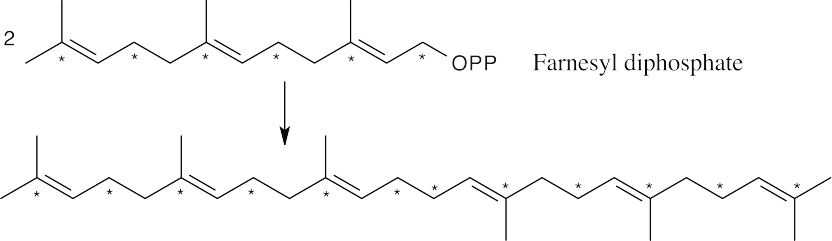
Farnesyl diphosphate (from the previous problem) dimerizes to form squalene.
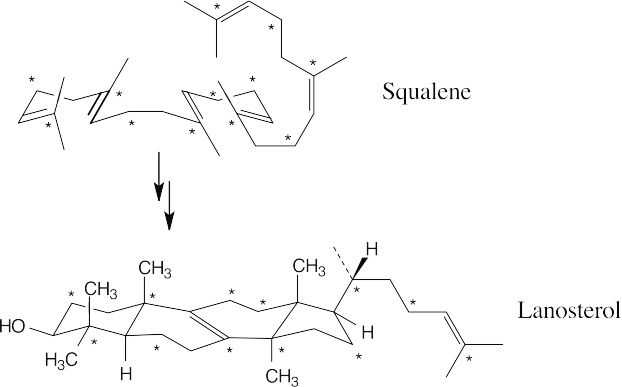
Squalene is converted to lanosterol by the series of steps pictured in Figure 27.15.
General Problems 27.34
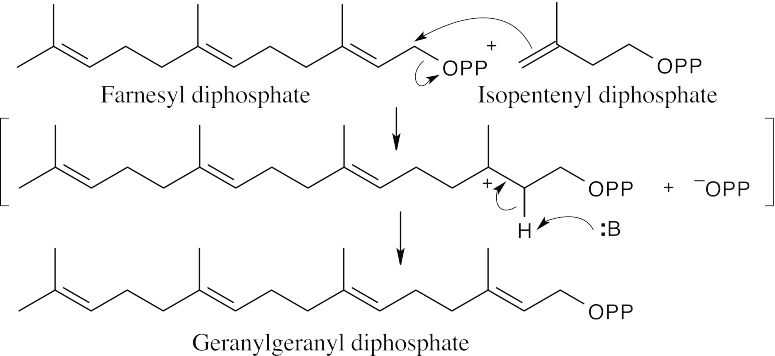
The precursor to flexibilene is formed from the reaction of farnesyl diphosphate and isopentenyl diphosphate.
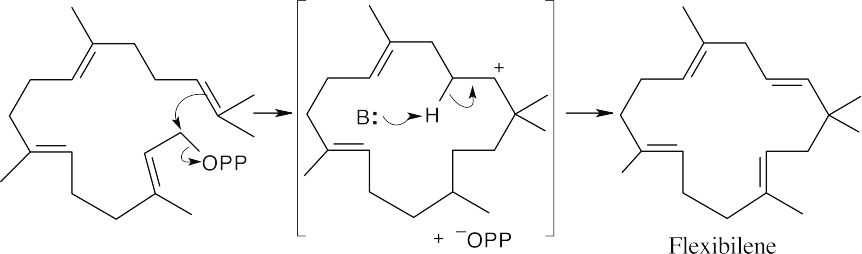
The precursor cyclizes by the now-familiar mechanism to produce flexibilene.
27.35
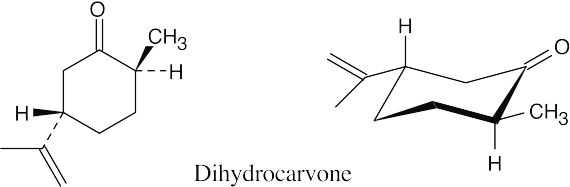
The two hydrocarbon substituents are equatorial in the most stable chair conformation.
27.36
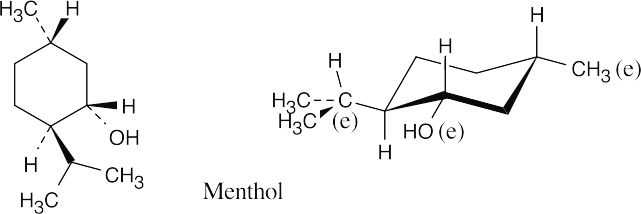
All ring substituents are equatorial in the most stable conformation of menthol.
27.37
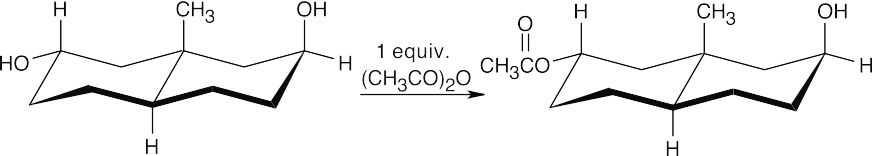 (a)
(a)
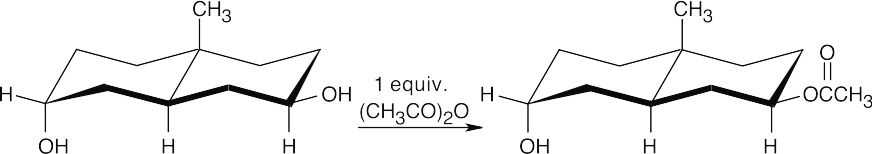 (b)
(b)
As always, use the stereochemistry of the groups at the ring junction to label the other substituents as equatorial or axial and then esterify the appropriate –OH group.
27.38
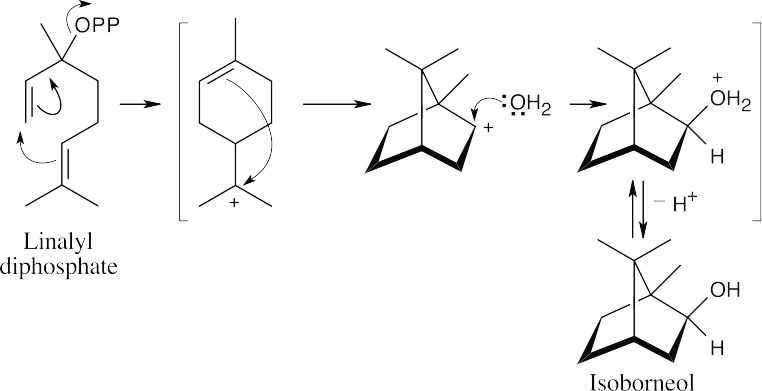
The initial addition is followed by a carbocation rearrangement to produce a secondary carbocation, which reacts with water to yield the secondary alcohol.
27.39
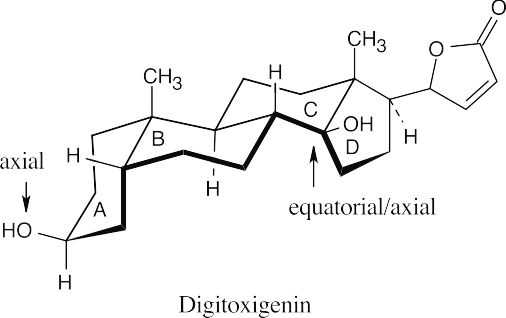
The hydroxyl group in ring A is axial, and the hydroxyl group at the ring C–D fusion is equatorial to ring C and axial to ring D. Notice that digitoxigenin has both an A–B cis ring fusion and a C–D cis ring fusion.
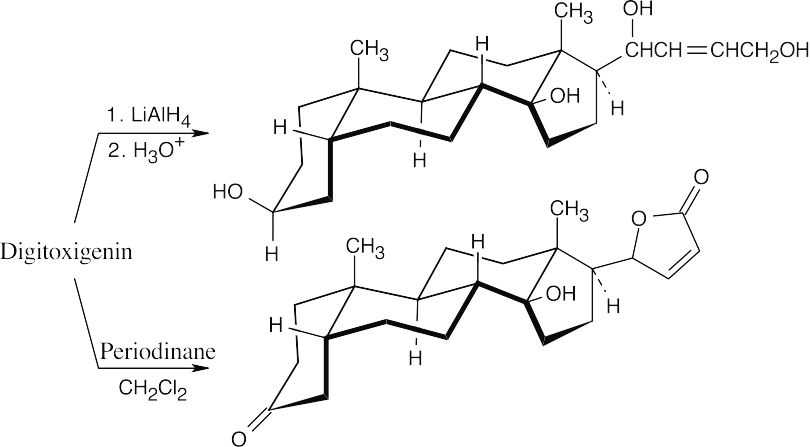
Lithium aluminum hydride reduces the lactone ring to a diol. Periodinane oxidizes only one hydroxyl group because the second group is tertiary.
27.41
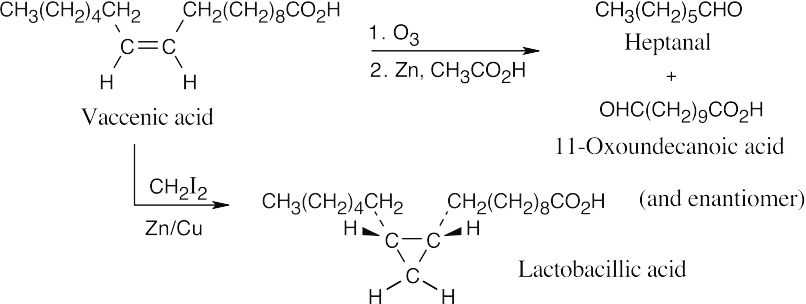
27.42
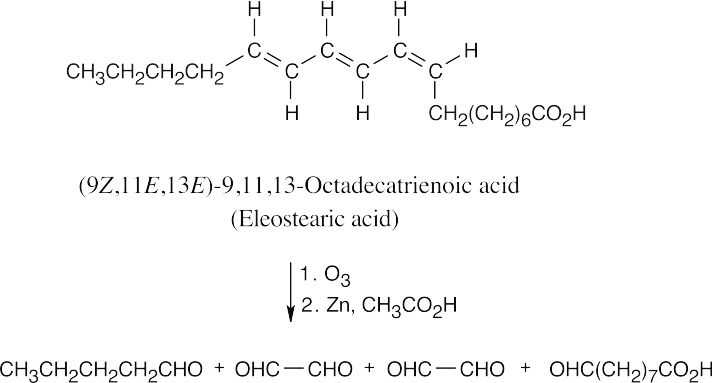
The stereochemistry of the double bonds can’t be determined from the information given.
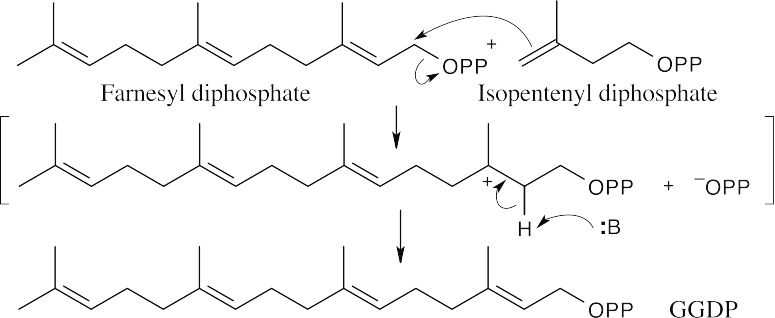 27.43 This mechanism also appears in Problem 27.32.
27.43 This mechanism also appears in Problem 27.32.
27.44
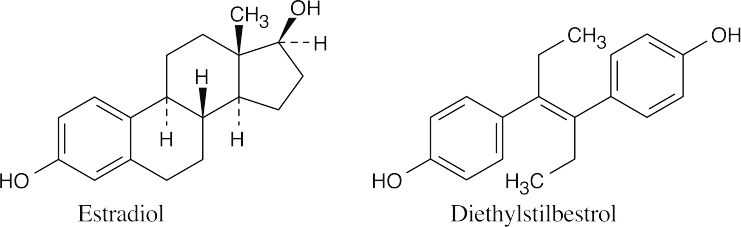
Estradiol and diethylstilbestrol resemble each other in having similar carbon skeletons, in having a phenolic ring, and in being diols.
27.45
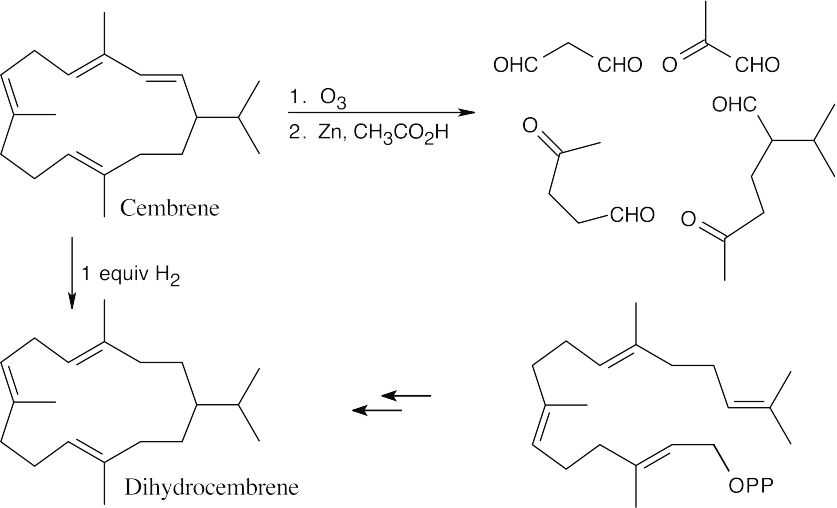
One equivalent of H2 hydrogenates the least substituted double bond. Dihydrocembrene has no ultraviolet absorption because it is not conjugated.
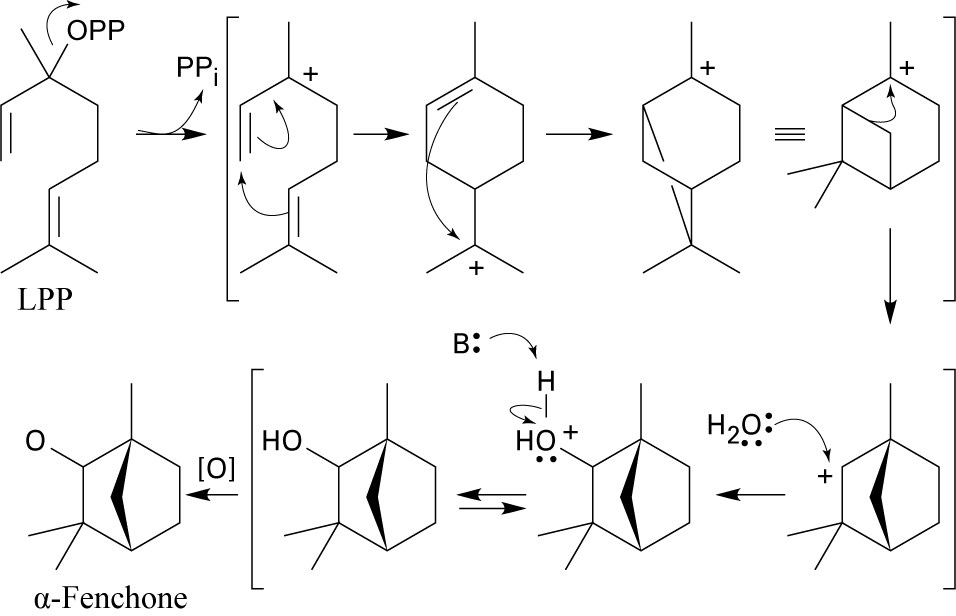
The mechanism follows the usual path: cyclization of linalyl diphosphate, followed by attack of the π electrons of the second double bond, produces an intermediate carbocation. A carbocation rearrangement occurs, and the resulting carbocation reacts with water to form an alcohol that is oxidized to give α-fenchone.
27.47
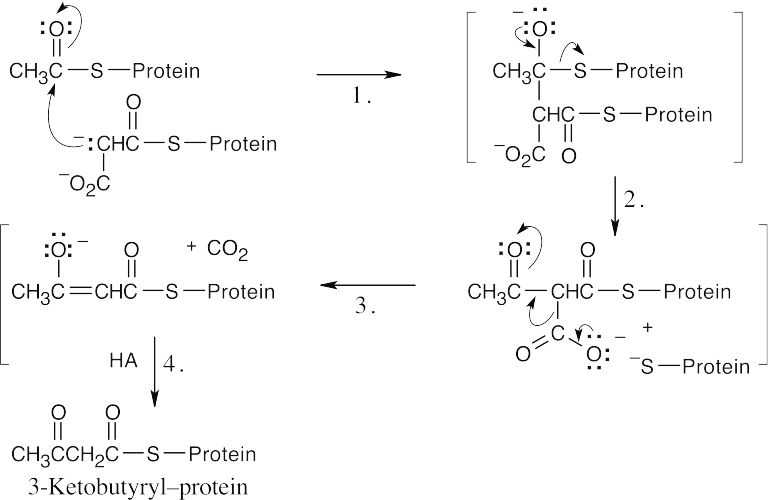
Step 1:Attack of malonyl–protein anion on acetyl–protein (Claisen condensation).
Step 2:Loss of S–protein.
Step 3:Decarboxylation.
Step 4:Protonation and tautomerization.
27.48
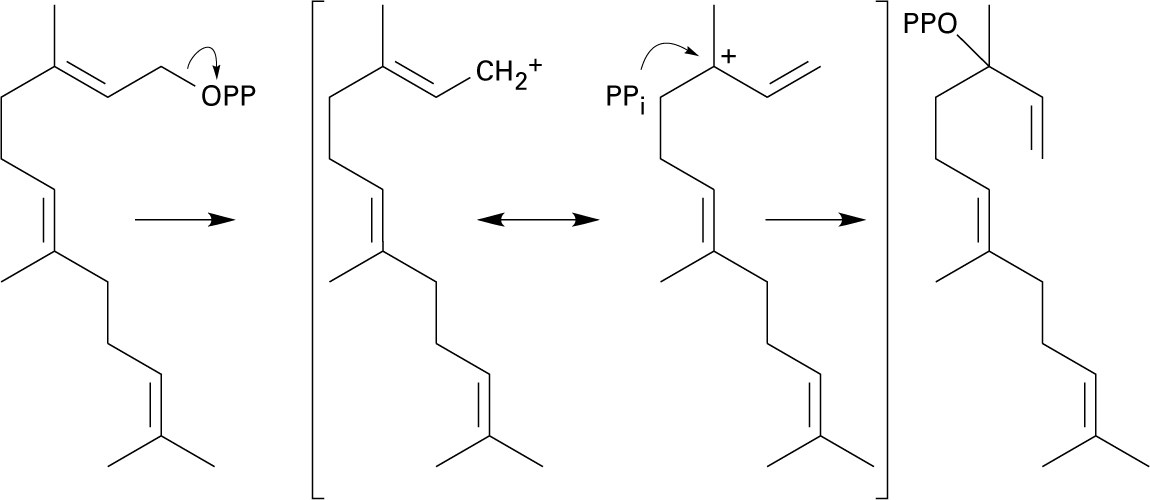
In this series of steps, dissociation of diphosphate ion allows bond isomerization to take place, making it possible for ring formation to occur. This mechanism is very similar to the mechanism shown in Figure 27.11.
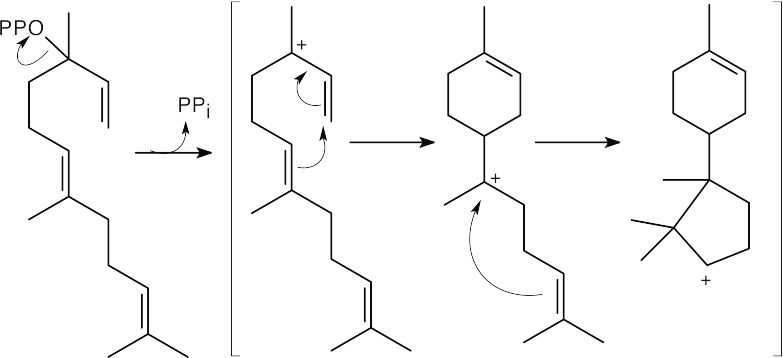
 Two cyclizations produce the trichodiene ring skeleton and a secondary carbocation.
Two cyclizations produce the trichodiene ring skeleton and a secondary carbocation.
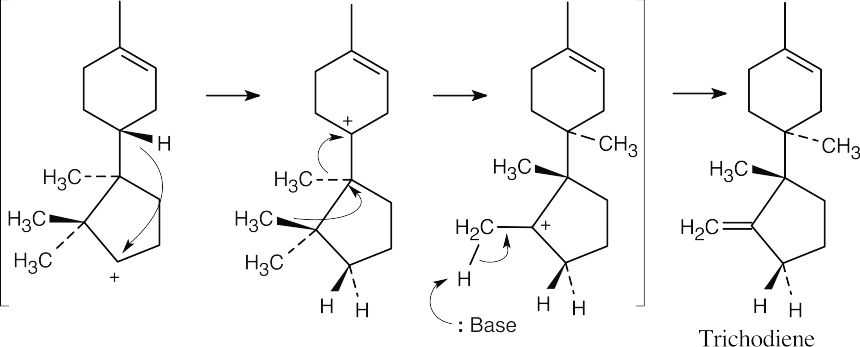
A hydride shift, two methyl shifts, and loss of –H+ yield trichodiene. This file is copyright 2023, Rice University. All Rights Reserved.

