30 Chapter 30 – Orbitals and Organic Chemistry: Pericyclic Reactions Solutions to Problems
30.1For ethylene:
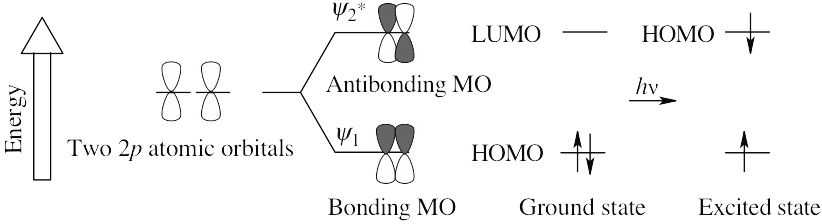
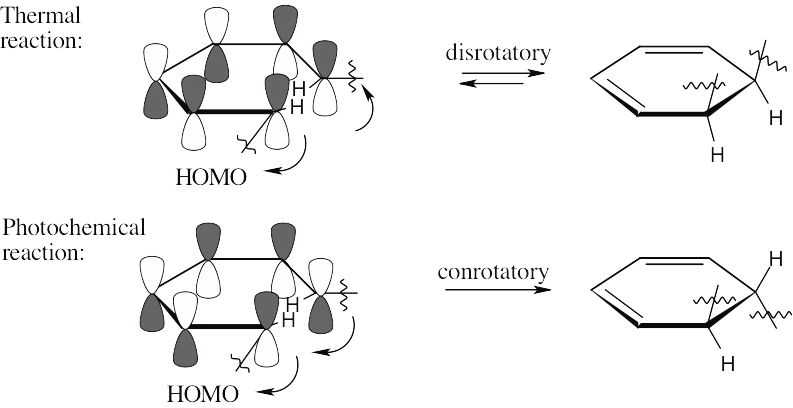
The two π electrons of ethylene occupy ψ1 in the ground state, making ψ1 the HOMO and ψ2* the LUMO. In the excited state, one electron occupies ψ1 and the other occupies ψ *, making ψ * the HOMO. Since all orbitals are occupied in the excited state, there is no LUMO.
22
For 1,3-butadiene:
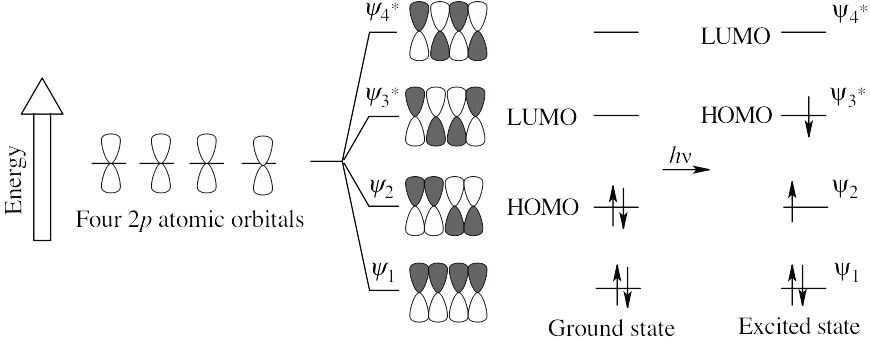
In the ground state, ψ2 is the HOMO, and ψ3* is the LUMO. In the excited state, ψ3* is the HOMO, and ψ4* is the LUMO.
30.2
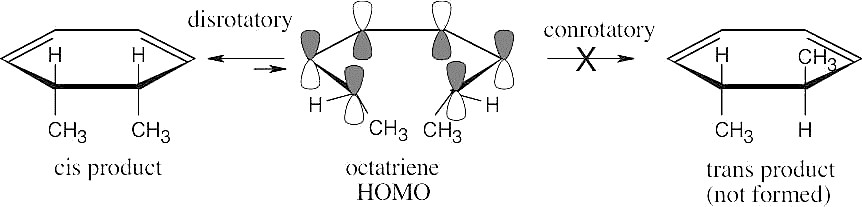
The symmetry of the octatriene HOMO predicts that ring closure occurs by a disrotatory path in the thermal reaction and that only cis product is formed.
30.3Note: Trans-3,4-dimethylcyclobutene is chiral; the S,S enantiomer will be used for this argument.
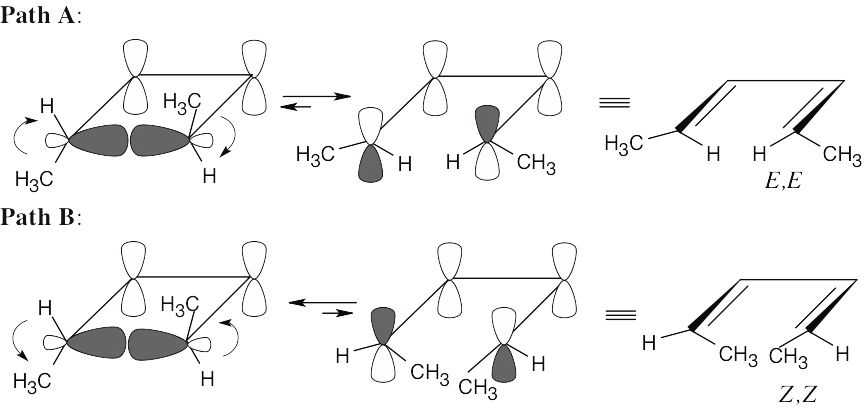
Conrotatory ring opening of trans-3,4-dimethylcyclobutene can occur in either a clockwise or a counterclockwise manner. Clockwise opening (path A) yields the E,E isomer; counterclockwise opening (path B) yields the Z,Z isomer. Production of (2Z,4Z)- 2,4-hexadiene is disfavored because of steric strain between the methyl groups in the transition state leading to ring-opened product.
30.4
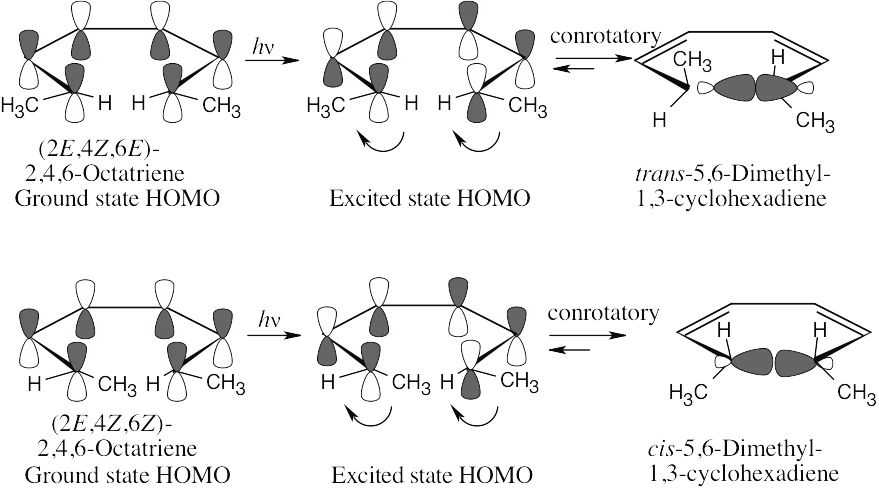
Photochemical electrocyclic reactions of 6 π electron systems always occur in a conrotatory manner.
30.5
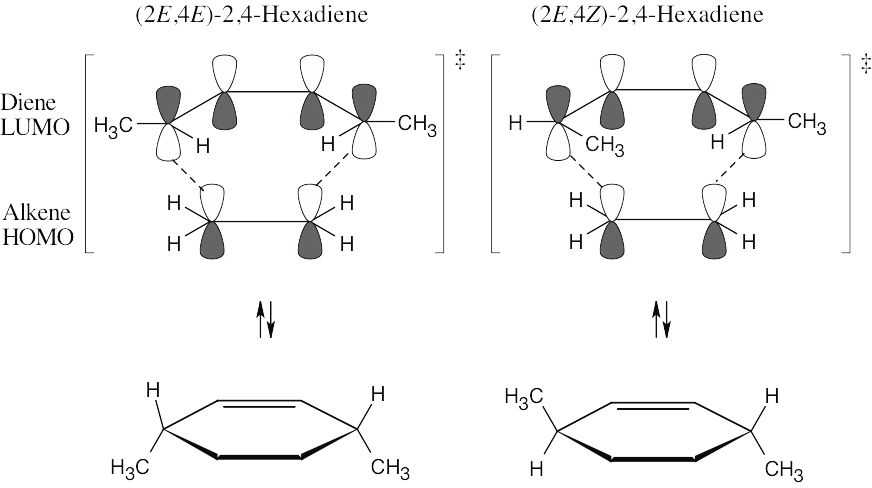
The Diels–Alder reaction is a thermal [4 + 2] cycloaddition, which occurs with suprafacial geometry. The stereochemistry of the diene is maintained in the product.
30.6
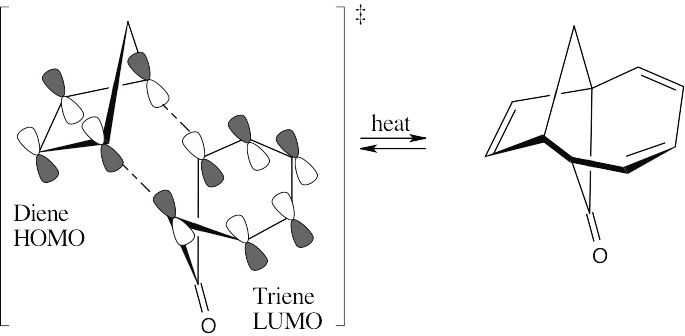
The reaction of cyclopentadiene and cycloheptatrienone is a [6 + 4] cycloaddition. This thermal cycloaddition proceeds with suprafacial geometry since five electron pairs are involved in the concerted process. The π electrons of the carbonyl group do not take part in the reaction.
30.7This [1,7] sigmatropic reaction proceeds with antarafacial geometry because four electron pairs are involved in the rearrangement.
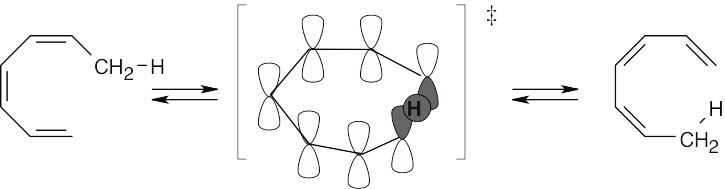
30.8
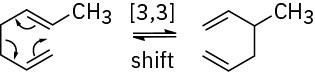
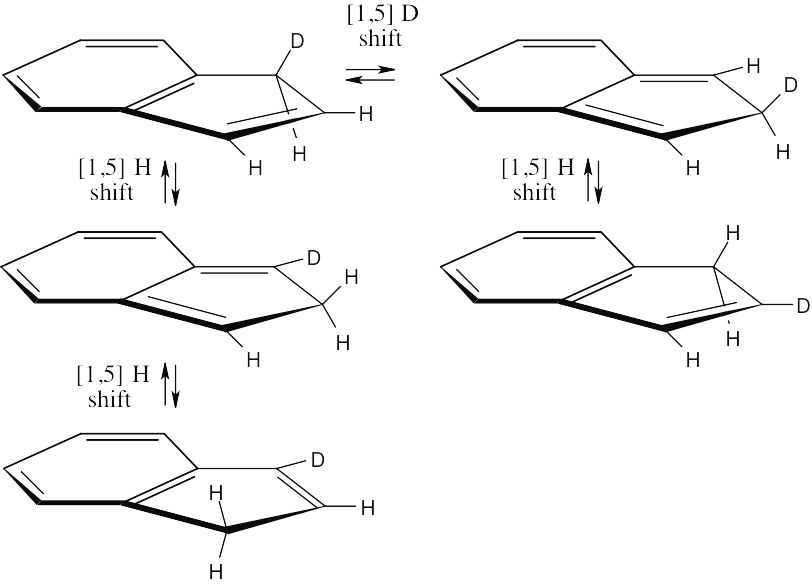
Scrambling of the deuterium label of 1-deuterioindene occurs by a series of [1,5] sigmatropic rearrangements. This thermal reaction involves three electron pairs – one pair of π electrons from the six-membered ring, the π electrons from the five-membered ring, and two electrons from a carbon-deuterium (or hydrogen) single bond – and proceeds with suprafacial geometry.
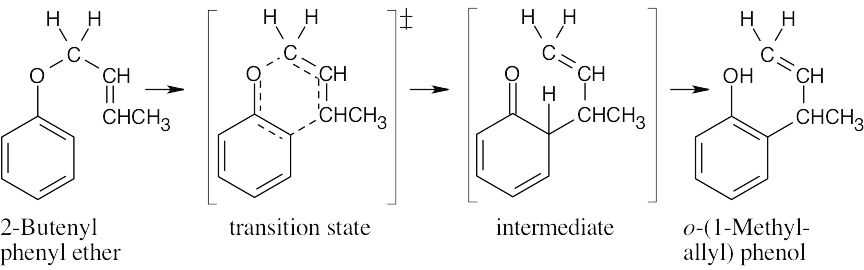
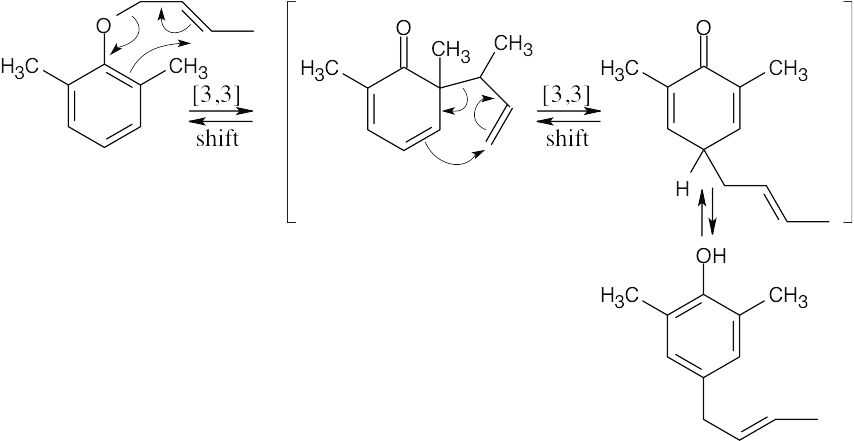
Draw the ether with the groups involved in the rearrangement positioned as they will appear in the product. Six bonds will either be broken or formed in the product; they are shown as dashed lines in the transition state. Redraw the bonds to arrive at the intermediate enone, which rearranges to the more stable phenol.
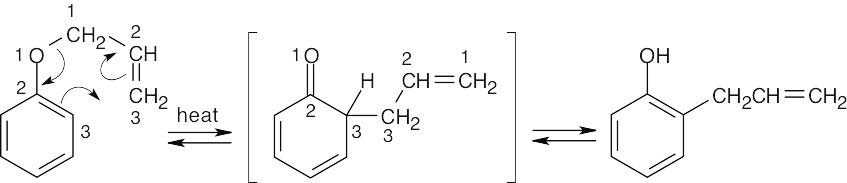
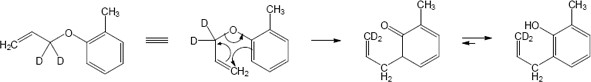
The Claisen rearrangement of an unsubstituted allyl phenyl ether is a [3,3] sigmatropic rearrangement in which the allyl group usually ends up in the position ortho to oxygen. In this problem, both ortho positions are occupied by methyl groups. The Claisen intermediate undergoes a second [3,3] rearrangement, and the final product is p-allyl phenol.
Type of reactionNumber of
electron pairs
Stereochemistry
Thermal electrocyclicfourconrotatory
Photochemical electrocyclicfourdisrotatory
Photochemical cycloadditionfoursuprafacial
Thermal cycloadditionfourantarafacial
Photochemical sigmatropic rearrangementfoursuprafacial
 Additional Problems Visualizing Chemistry 30.13
Additional Problems Visualizing Chemistry 30.13
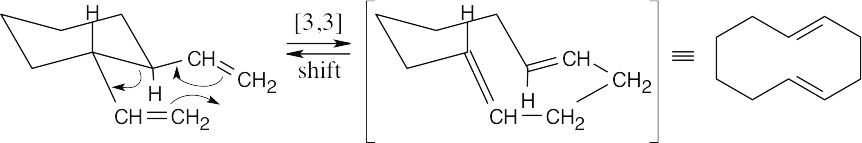
This reaction is a [3,3] sigmatropic rearrangement that yields 1,5-cyclodecadiene as a product.
30.14
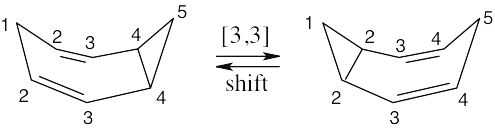
The 13C NMR spectrum of homotropilidene would show five peaks if rearrangement were slow. In fact, rearrangement occurs at a rate that is too fast for NMR to detect. The 13C NMR spectrum taken at room temperature is an average of the two equilibrating forms, in which positions 1 and 5 are equivalent, as are positions 2 and 4. Thus, only three distinct types of carbons are visible in the 13C NMR spectrum of homotropilidene.
 Mechanism Problems 30.15
Mechanism Problems 30.15
30.16 (a)
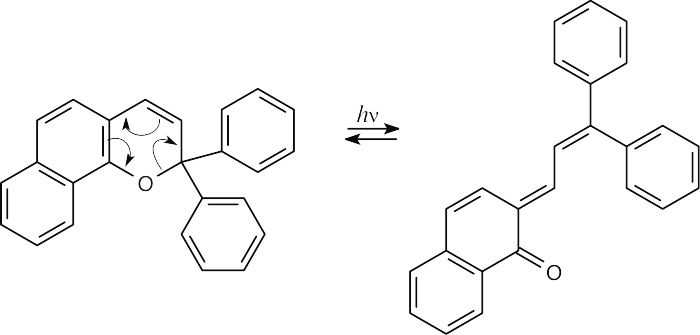
(b)The rearrangement product absorbs at a longer wavelength because it has a more extensive system of conjugated double bonds.
30.17
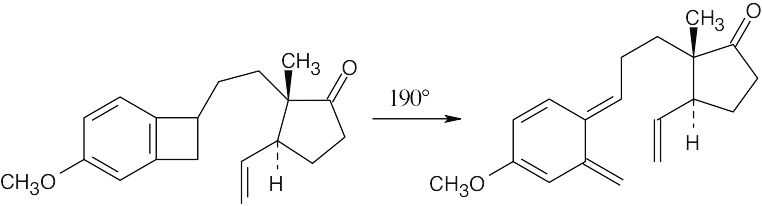
The first reaction is an electrocyclic opening of a cyclobutene ring.
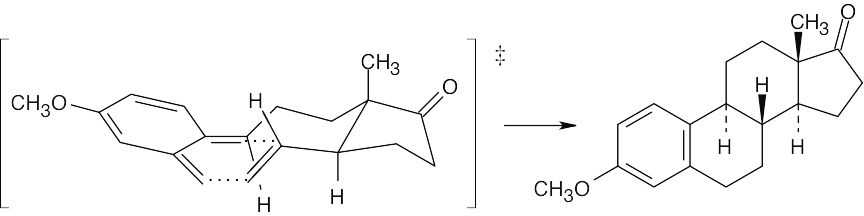
Formation of estrone methyl ether occurs by a Diels–Alder [4 + 2] cycloaddition.
30.18
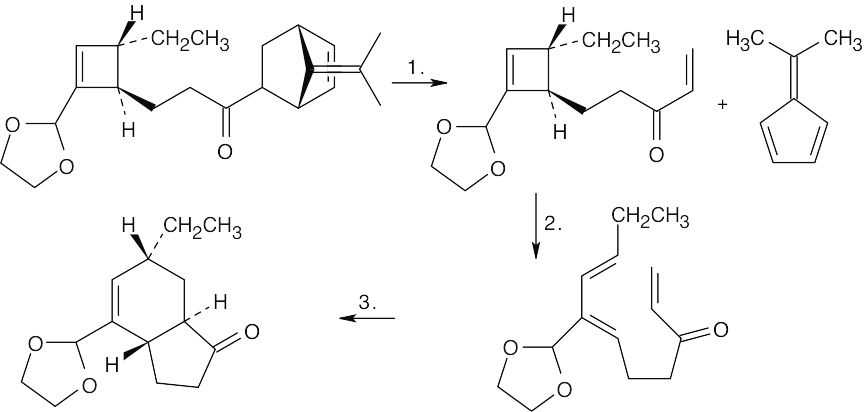
Reaction 1:Reverse Diels–Alder [4 + 2] cycloaddition;
Reaction 2:Conrotatory electrocyclic opening of a cyclobutene ring;
Reaction 3:Diels–Alder [4 + 2] cycloaddition.
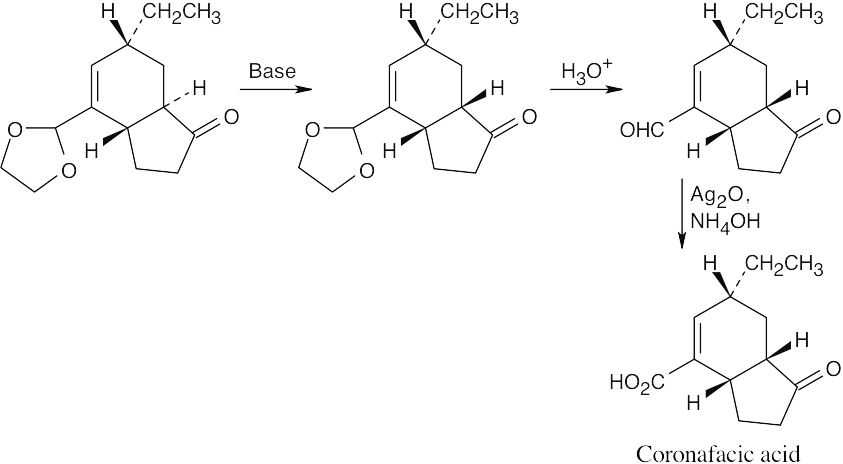
Treatment with base enolizes the ketone and changes the ring junction from trans to cis. A cis ring fusion is less strained when a six-membered ring is fused to a five-membered ring.
30.19
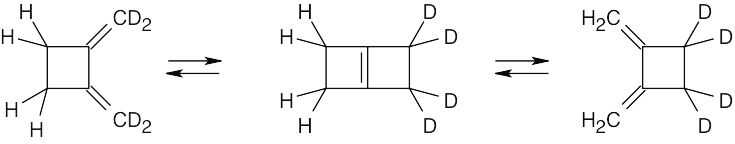
Each of the two electrocyclic reactions involves two pairs of electrons and proceeds in a conrotatory manner.
Electrocyclic Reactions
30.20 (a)
Rotation of the orbitals in the 6 π electron system occurs in a disrotatory fashion. According to the rules in Table 30.1, the reaction should be carried out under thermal conditions.
 (b)
(b)
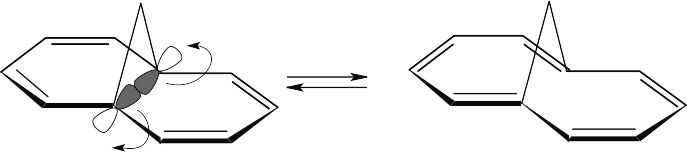
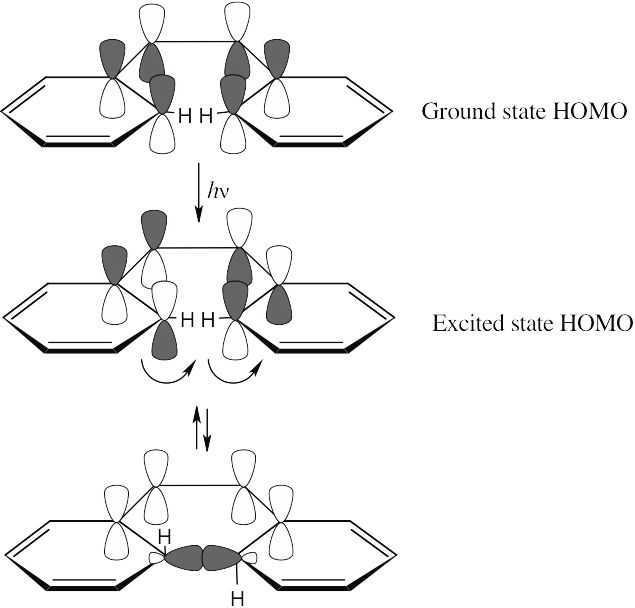
For the hydrogens to be trans in the product, rotation must occur in a conrotatory manner. This can happen only if the HOMO has the symmetry pictured. For a 6 π electron system, this HOMO must arise from photochemical excitation of a π electron. To obtain a product having the correct stereochemistry, the reaction must be carried out under photochemical conditions.
The diene can cyclize by either of two conrotatory paths to form cyclobutenes A and B.
Opening of each cyclobutene ring can occur by either of two conrotatory routes to yield the isomeric dienes. Using B as an example:
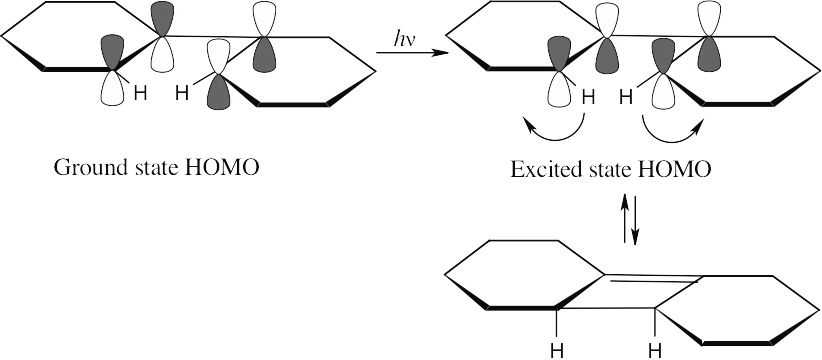
A photochemical electrocyclic reaction involving two electron pairs proceeds in a
disrotatory manner (Table 30.1).
The two hydrogen atoms in the four-membered ring are cis to each other in the cyclobutene product.
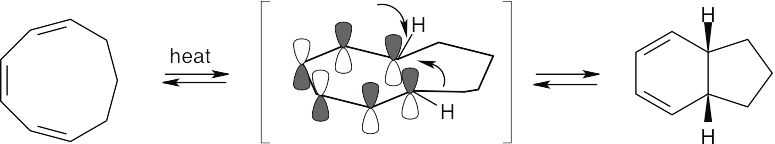 The cyclononatriene is a 6 π electron system that cyclizes by a disrotatory route under thermal conditions. The two hydrogens at the ring junction have a cis relationship.
The cyclononatriene is a 6 π electron system that cyclizes by a disrotatory route under thermal conditions. The two hydrogens at the ring junction have a cis relationship.
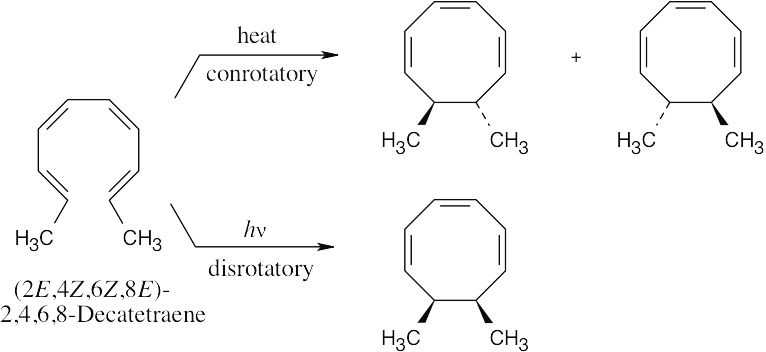
Four electron pairs undergo reorganization in this electrocyclic reaction. The thermal reaction occurs with conrotatory motion to yield a pair of enantiomeric trans-7,8- dimethyl-1,3,5-cyclooctatrienes. The photochemical cyclization occurs with disrotatory motion to yield the cis-7,8-dimethyl isomer.
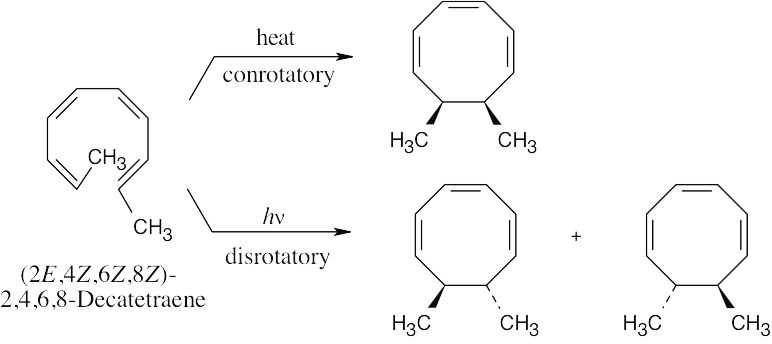
Two electrocyclic reactions, involving three electron pairs each, occur in this isomerization. The thermal reaction is a disrotatory process that yields two cis-fused six- membered rings. The photochemical reaction yields the trans-fused isomer. The two pairs of π electrons in the eight-membered ring do not take part in the electrocyclic reaction.
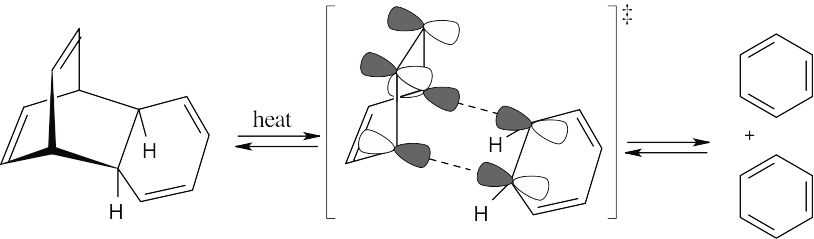 Cycloaddition Reactions 30.27
Cycloaddition Reactions 30.27
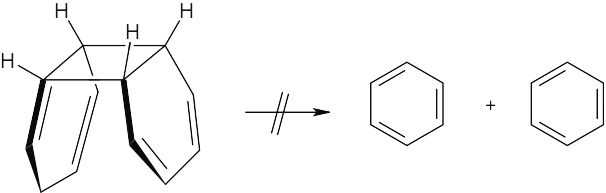 This reaction is a reverse [4 + 2] cycloaddition. The reacting orbitals have the correct symmetry for the reaction to take place by a favorable suprafacial process.
This reaction is a reverse [4 + 2] cycloaddition. The reacting orbitals have the correct symmetry for the reaction to take place by a favorable suprafacial process.
This [2 + 2] reverse cycloaddition is not likely to occur as a concerted process because the antarafacial geometry required for the thermal reaction is not possible for a four π– electron system.
30.28
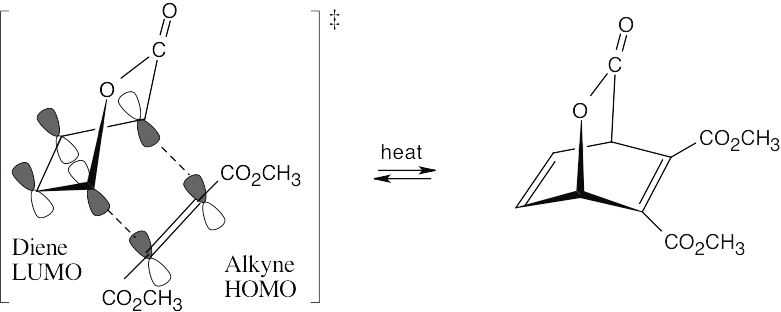
Formation of the bicyclic ring system occurs by a suprafacial [4 + 2] Diels–Alder cycloaddition process. Only one pair of π electrons from the alkyne is involved in the reaction; the carbonyl π electrons are not involved.
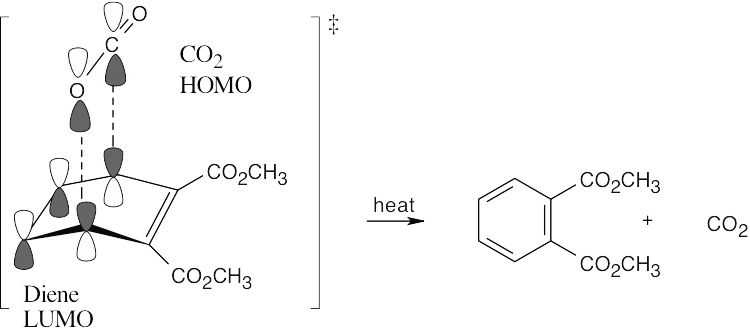
30.29
The first reaction is a Diels–Alder [4 + 2] cycloaddition, which proceeds with suprafacial geometry.
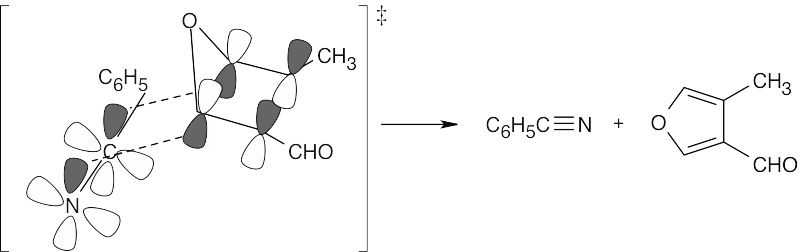
The second reaction is a reverse Diels–Alder [4 + 2] cycloaddition.
 Sigmatropic Rearrangements 30.30
Sigmatropic Rearrangements 30.30
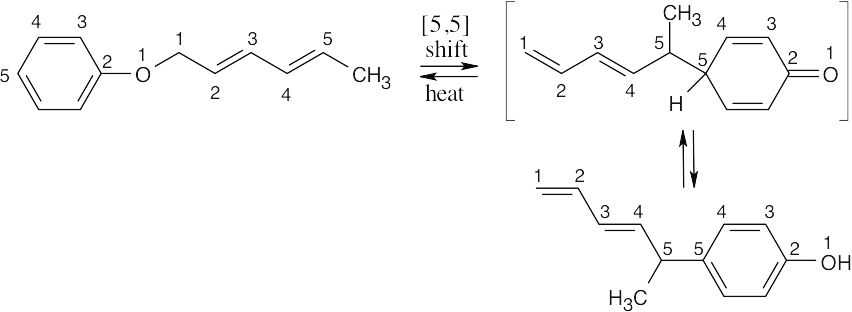
This thermal sigmatropic rearrangement is a suprafacial process since five electron pairs are involved in the reaction.
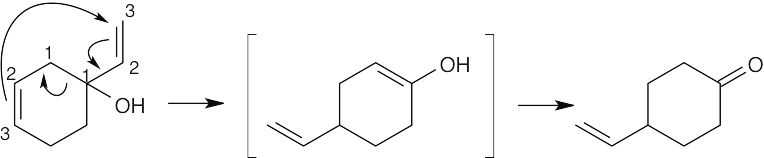
30.31 The product of this [3,3] sigmatropic rearrangement is an enol that tautomerizes to a ketone.
30.32
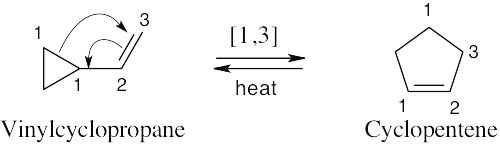
This reaction is a [1,3] sigmatropic rearrangement.
30.33
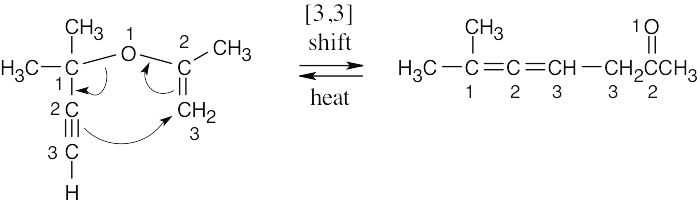
An allene is formed by a [3,3] sigmatropic rearrangement.
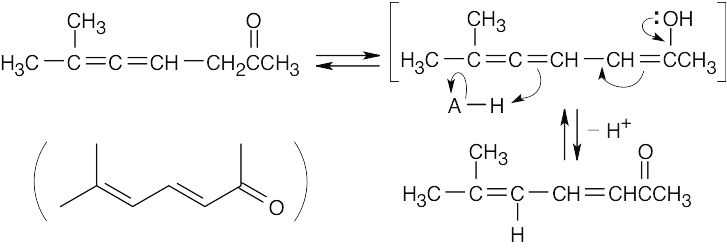
Acid catalyzes isomerization of the allene to a conjugated dienone via an intermediate enol.
30.34
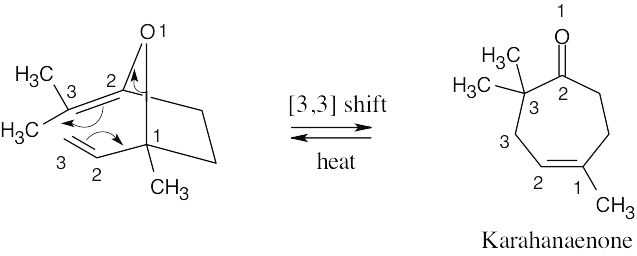
Karahanaenone is formed by a [3,3] sigmatropic rearrangement (Claisen rearrangement).
General Problems
Tables 30.1–30.3 may be helpful. The first step is always to find the number of electron pairs involved in the reaction.
Type of reactionNumber of
electron pairs
Stereochemistry
Photochemical [1,5] sigmatropic rearrangement3antarafacial
Thermal [4 + 6] cycloaddition5suprafacial
Thermal [1,7] sigmatropic rearrangement4antarafacial
Photochemical [2 + 6] cycloaddition4suprafacial
Ring opening of Dewar benzene is a process involving two electron pairs and, according to Table 30.1, should occur by a conrotatory pathway. However, if you look back to other ring openings of cis-fused cyclobutenes, you will see that conrotatory ring opening produces a diene in which one of the double bonds is trans. Since a trans double bond in a six-membered ring is not likely to be formed, ring opening occurs by a different, higher energy, nonconcerted pathway.
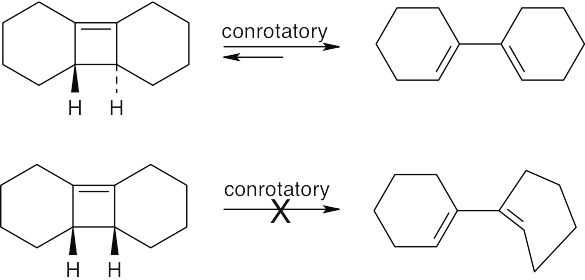
Ring opening of the trans-cyclobutene isomer proceeds by the expected conrotatory route to form the observed product. For the cis-cyclobutene isomer, the observed product can be formed by a four-electron pericyclic process only if the four-membered ring geometry is trans. Ring opening of the cis isomer by a concerted process would form a severely strained six-membered ring containing a trans double bond. Reaction of the cis isomer to yield the observed product occurs instead by a higher energy, nonconcerted path.
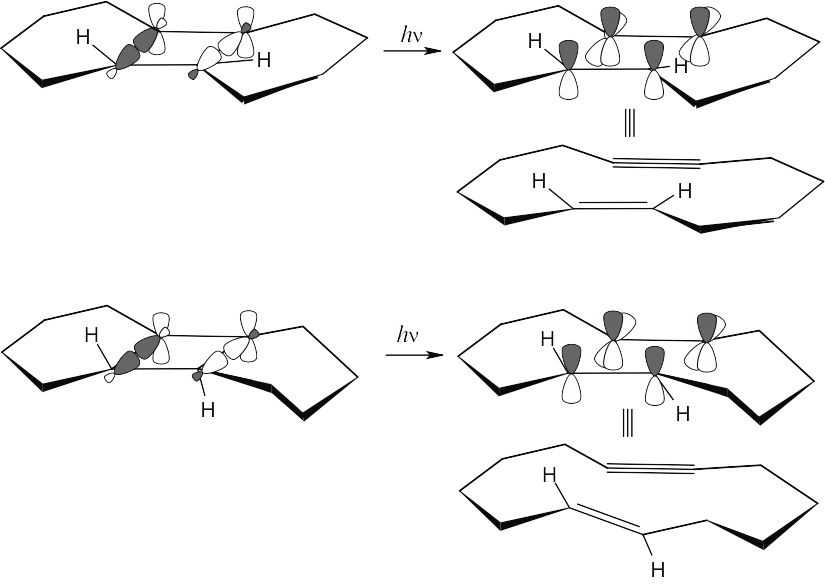
Both reactions are [2 + 2] photochemical electrocyclic reactions, which occur with disrotatory motion.
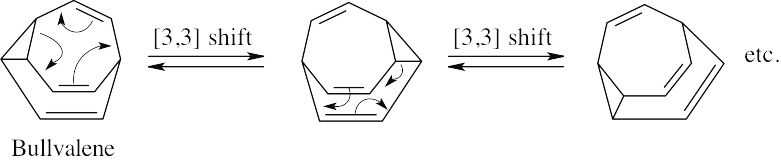
Bullvalene can undergo [3,3] sigmatropic rearrangements in all directions. At 100 °C, the rate of rearrangement is fast enough to make all hydrogen atoms equivalent, and only one signal is seen in the 1H NMR spectrum.
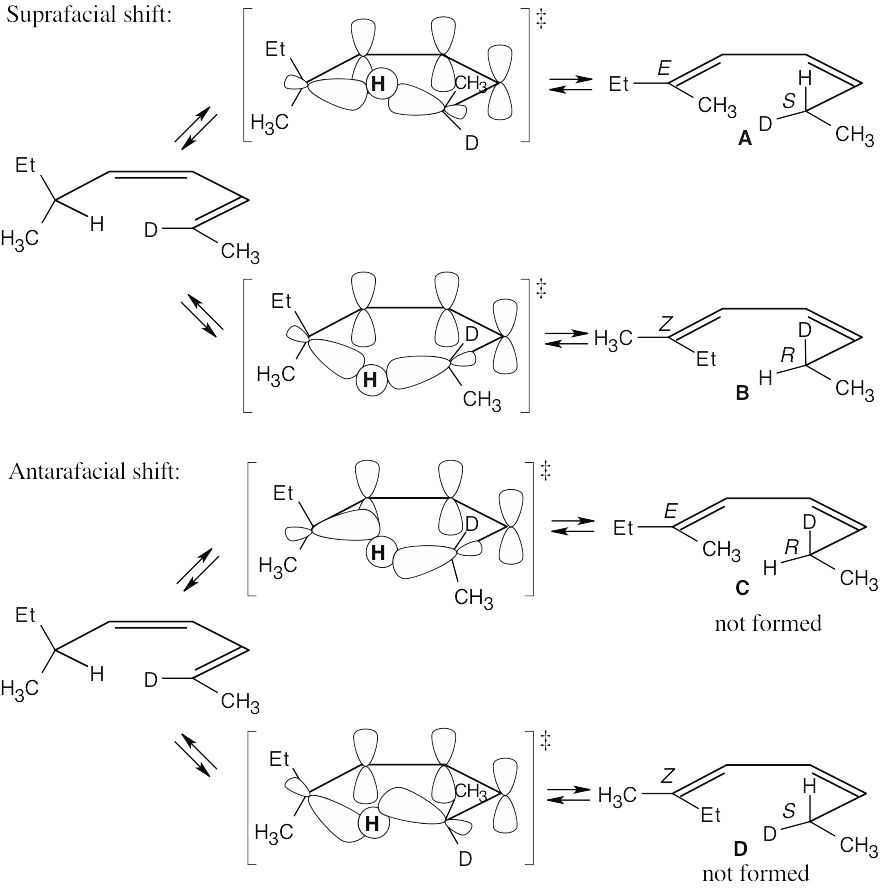
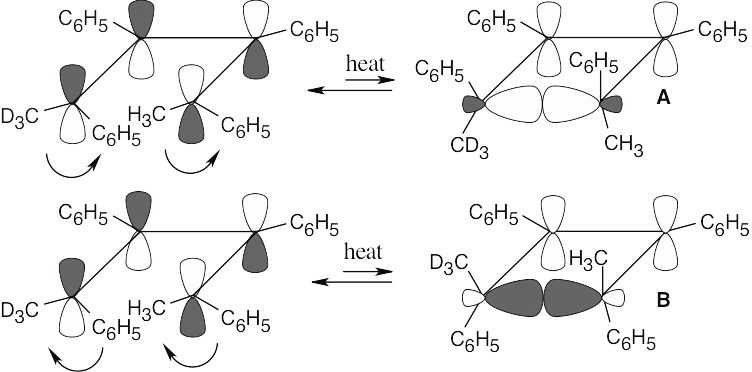
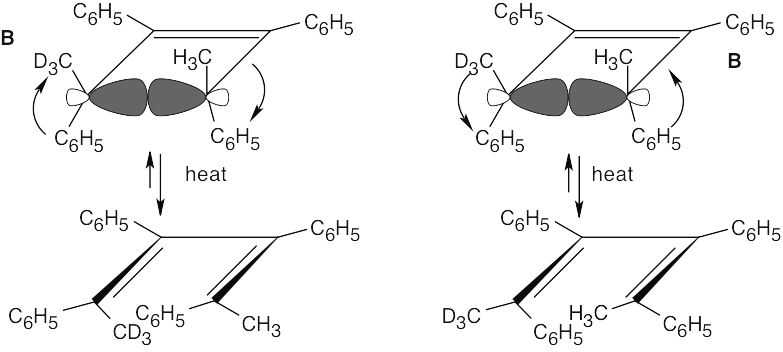
The observed products A and B result from a [1,5] sigmatropic hydrogen shift with suprafacial geometry, and they confirm the predictions of orbital symmetry. C and D are not formed.
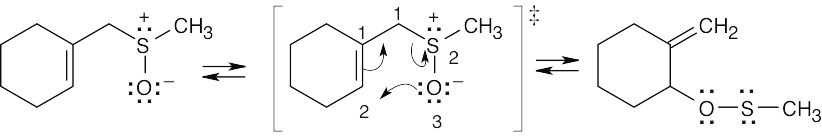
This [2,3] sigmatropic rearrangement involves three electron pairs and should occur with suprafacial geometry.
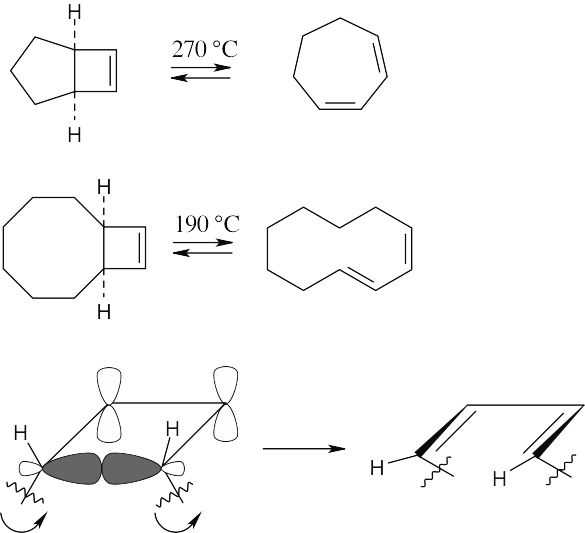
Concerted thermal ring opening of a cis-fused cyclobutene is conrotatory and yields a product having one cis and one trans double bond. The ten-membered ring product of reaction 2 is large enough to accommodate a trans double bond, but a seven-membered ring containing a trans double bond is highly strained. Opening of the cyclobutene ring in reaction 1 occurs by a higher energy nonconcerted process to yield a seven-membered ring having two cis double bonds.
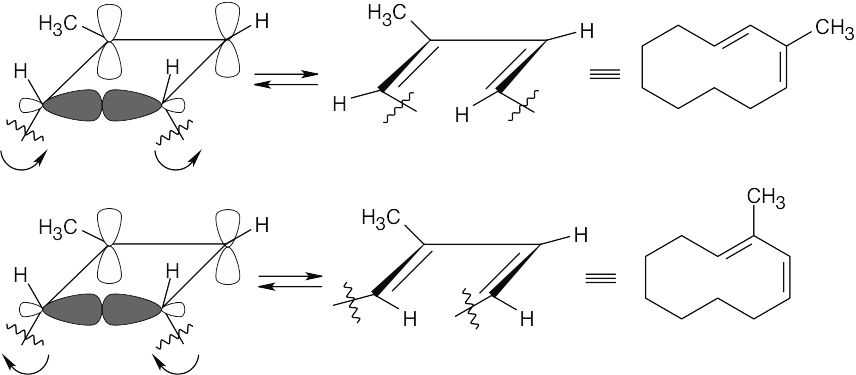
Thermal ring opening of the methylcyclobutene ring can occur by either of two symmetry-allowed conrotatory paths to yield the observed product mixture.
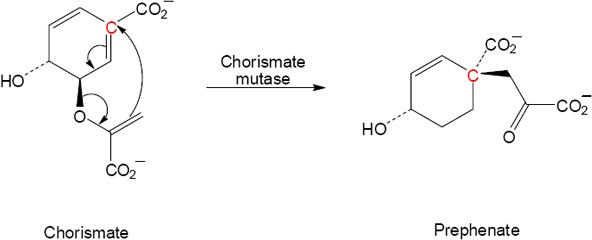
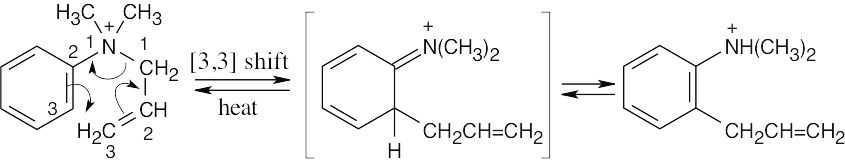
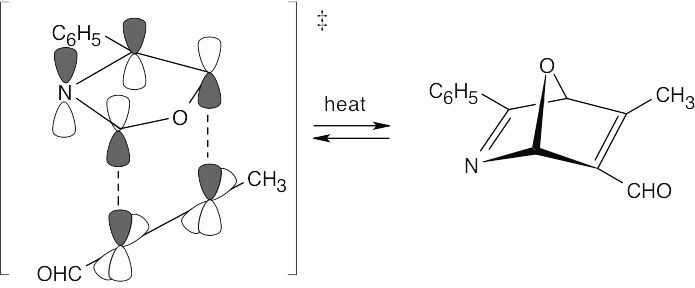
Note: For these problems it is best to redraw the molecule so that one can see the six atoms where the electrons will flow as a ring.
In this case the original Claisen rearrangement product will further tautomerize to a more stable phenol.
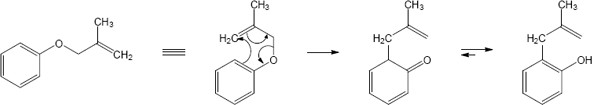
(b)
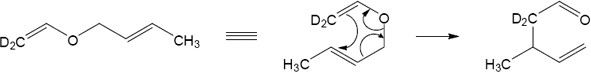
In this case the original Claisen rearrangement product will further tautomerize to a more stable phenol.
This file is copyright 2023, Rice University. All Rights Reserved.

