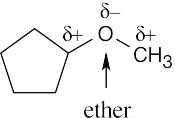
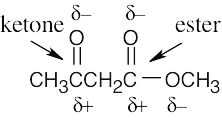
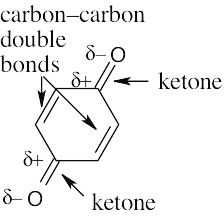
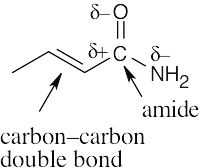
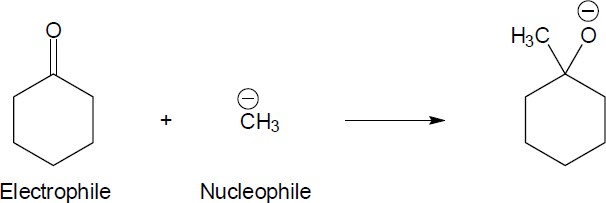
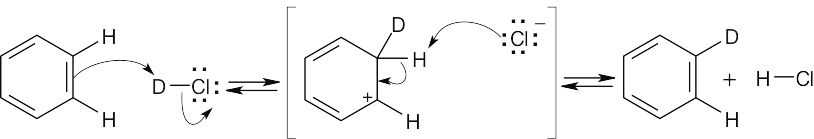
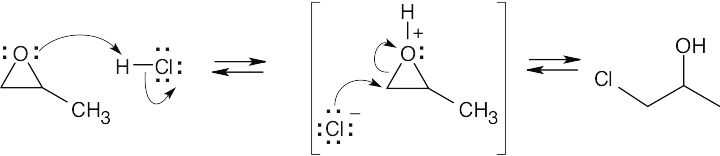
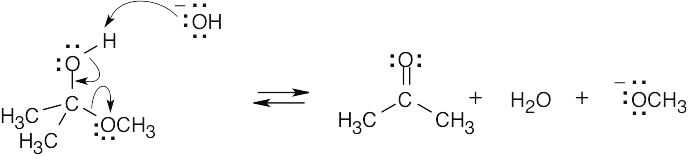
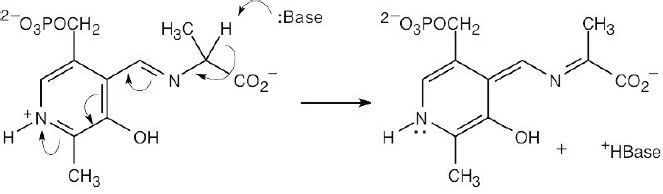
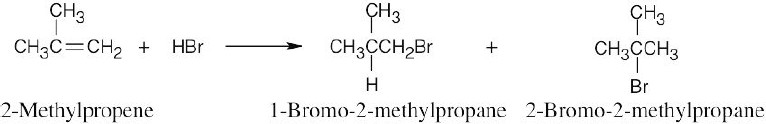
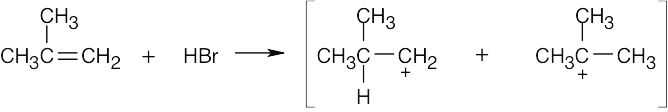
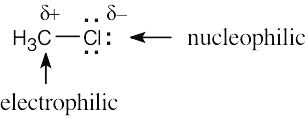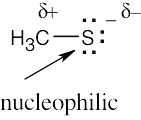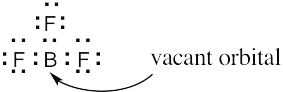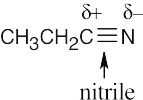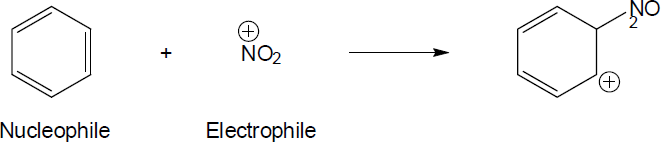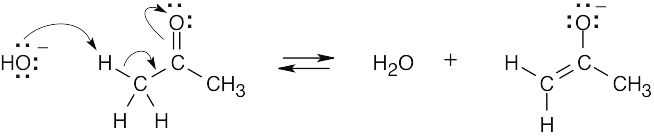6 Chapter 6 – An Overview of Organic Reactions Solutions to Problems
Chapter 6 – An Overview of Organic Reactions
Solutions to Problems
| 6.1 |
|
| 6.2 | Keep in mind:
|
| 6.3 | BF3 is likely to be an electrophile because the electrostatic potential map indicates that it is electron-poor (blue). The electron-dot structure shows that BF3 lacks a complete electron octet and can accept an electron pair from a nucleophile.
|
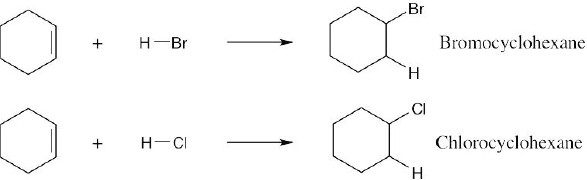
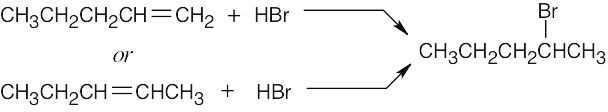
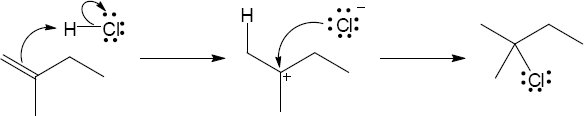
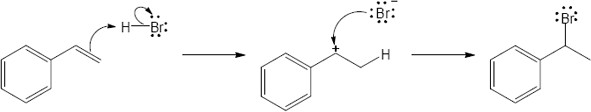
| 6.4 | Reaction of cyclohexene with HCl or HBr is an electrophilic addition reaction in which halogen acid adds to a double bond to produce a haloalkane.
|
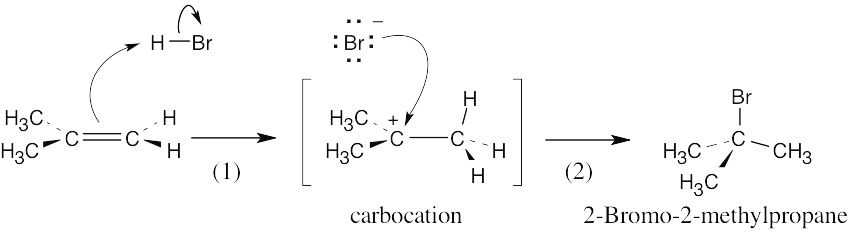
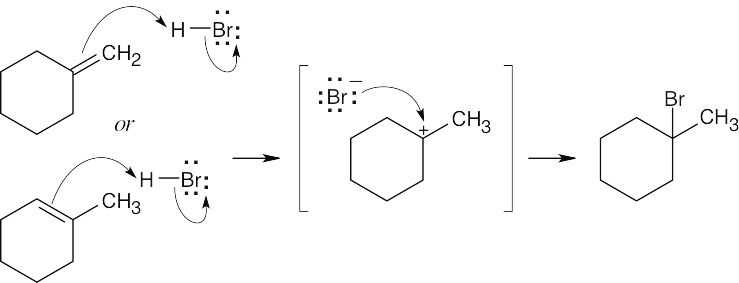
| 6.5 | The mechanism is pictured in Figure 6.4.
The steps: (1) Attack of the π electrons of the double bond on HBr, forming a carbocation (2) Formation of a C–Br bond by electron pair donation from Br– to form the neutral addition product
|
| 6.6 | For curved arrow problems, follow these steps:
(1) Locate the bonding changes. In (a), a bond from nitrogen to chlorine has formed, and a Cl-Cl bond has broken. (2) Identify the nucleophile and electrophile (in (a), the nucleophile is ammonia and the electrophile is one Cl in the Cl2 molecule), and draw a curved arrow whose tail is near the nucleophile and whose head is near the electrophile. (3) Check to see that all bonding changes are accounted for. In (a), we must draw a second arrow to show the unsymmetrical bond-breaking of Cl2 to form Cl–.
|
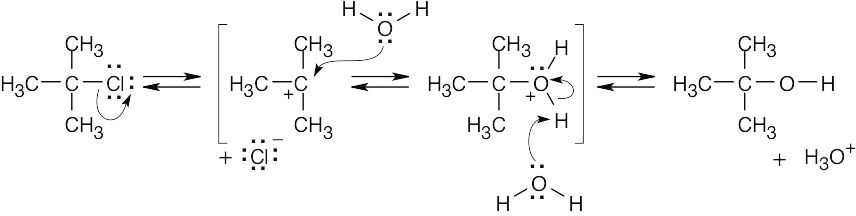
| 6.7 | This mechanism will be studied in a later chapter.
|
| 6.8 |
|
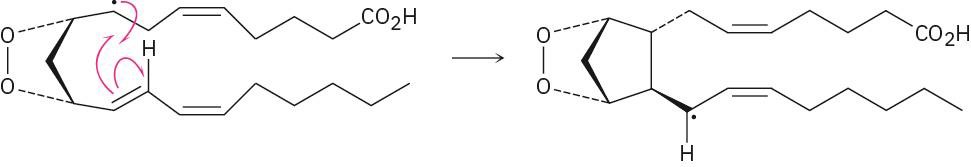
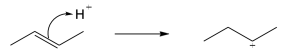
| 6.9 | Even though this molecule is complex, concentrate on the bonds formed and the bonds broken. The tails of the arrows show the location of the bond to be broken, and the heads show where the electrons are moving. In radical reactions, the arrow is a fishhook (half- headed).
The reaction is a radical addition to a double bond and is a rearrangement. |
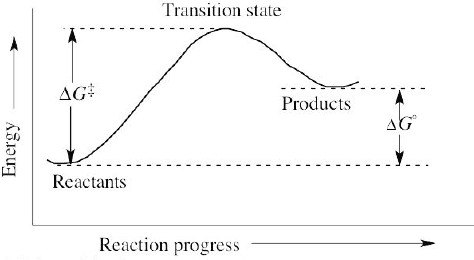
| 6.10 | A negative value of ΔG° indicates that a reaction is favorable. Thus, a reaction with ΔG° = –44 kJ/mol is more favorable than a reaction with ΔG° = +44 kJ/mol. |
| 6.11 | From the expression ΔG° = –RT lnKeq, we can see that a large Keq is related to a large negative ΔG° and a favorable reaction. Consequently, a reaction with Keq = 1000 is more exergonic than a reaction with Keq = 0.001. |
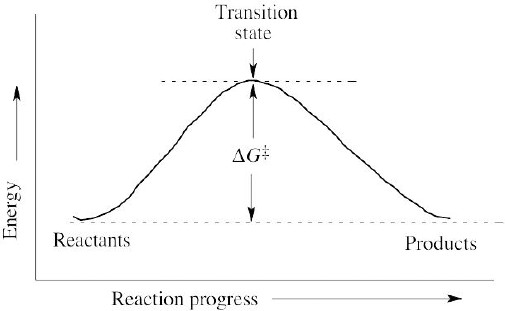
| 6.12 | A reaction with ΔG‡ = 45 kJ/mol is faster than a reaction with ΔG‡ = 70 kJ/mol because a larger value for ΔG‡ indicates a slower reaction. |
| 6.13 |
|
Additional Problems
Visualizing Chemistry
| 6.14 |
|
| 6.15 |
|
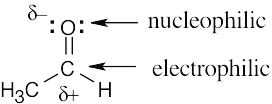
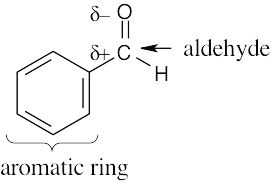
| 6.16 | (a) | The electrostatic potential map shows that the formaldehyde oxygen is electron-rich, and the carbon–oxygen bond is polarized. The carbon atom is thus relatively electron- poor and is likely to be electrophilic. |
| (b) | The sulfur atom is more electron-rich than the other atoms of methanethiol and is likely to be nucleophilic. |
| 6.17 |
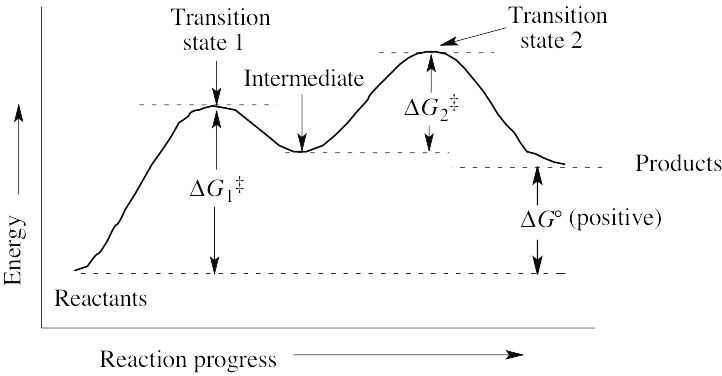
(a) ΔG° is positive. (b) There are two steps in the reaction. (c) There are two transition states, as indicated on the diagram. |
| 6.18 |
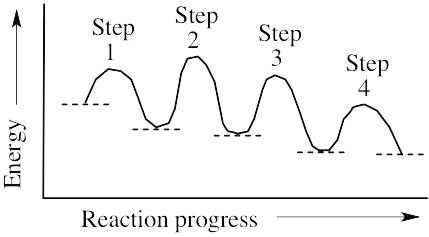
(a) The reaction involves four steps, noted above. (b) Step 1 is the most exergonic because the energy difference between reactant and product (ΔG°) is greatest. (c) Step 2 is slowest because it has the largest value of ΔG‡. |
Energy Diagrams and Reaction Mechanisms
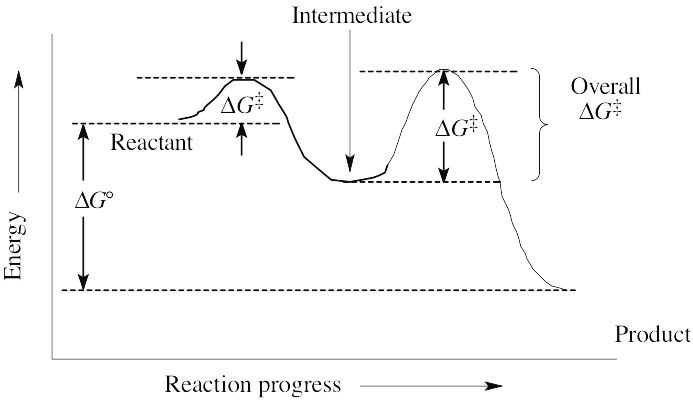
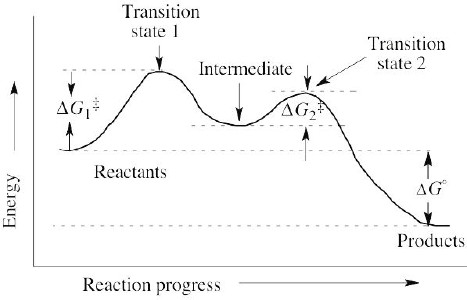
| 6.19 | A transition state represents a structure occurring at an energy maximum. An intermediate occurs at an energy minimum between two transition states. Even though an intermediate may be of such high energy that it cannot be isolated, it is still of lower energy than the transition states surrounding it. |
| 6.20 |
ΔG° is positive because Keq < 1. |
| 6.21 |
ΔG° is negative because Keq > 1. |
| 6.22 | Problem 6.28 shows a reaction energy diagram of a two-step exergonic reaction. Step 2 is faster than step 1 because ΔG‡2 < ΔG‡1. |
| 6.23 |
A reaction with Keq = 1 has ΔG° = 0. |
| 6.24 | (a) | The reaction is exothermic because the sign of ΔH° is negative. |
| (b) | ΔG° = ΔH° – TΔS°
= –44 kJ/mol – (298 K) [–0.12 kJ/(K·mol)] = –44 kJ/mol + 36 kJ/mol = –8 kJ/mol |
|
| The reaction is favorable because the sign of ΔG° is negative. |
| 6.25 | (a) | 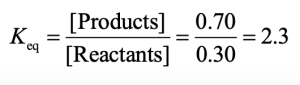 |
| (b) | Section 6.9 states that reactions that occur spontaneously have ΔG‡ of less than 80 kJ/mol at room temperature. Since this reaction proceeds slowly at room temperature, ΔG‡ is probably close to 80 kJ/mol. | |
| (c) | 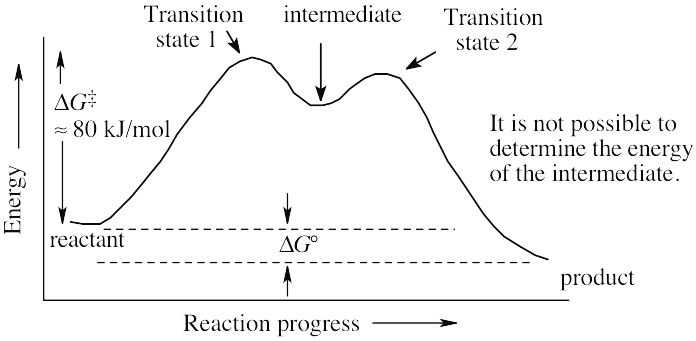 |
| 6.26 |
|
| 6.27 | (a) Mechanism:
(b) Mechanism:
(c) Mechanism:
|
| 6.28 | (a) | 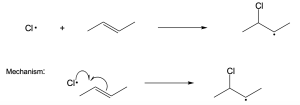 |
| (b) | 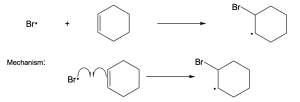 |
|
| (c) | 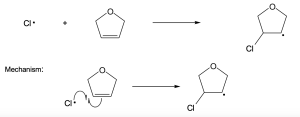 |
Polar Reactions
| 6.29 |
|
| 6.30 | (a) | The reaction between bromoethane and sodium cyanide is a substitution because two reagents exchange parts. |
| (b) | This reaction is an elimination because two products (cyclohexene and H2O) are produced from one reactant | |
| (c) | Two reactants form one product in this addition reaction. | |
| (d) | This is a substitution reaction. |
| 6.31 |
|
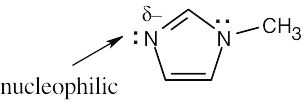
| 6.32 | Nucleophiles are the electron donors in carbon bond forming reactions while the electron acceptors are the electrophiles.
|
| 6.33 |
|
| 6.34 |
|
Radical Reactions
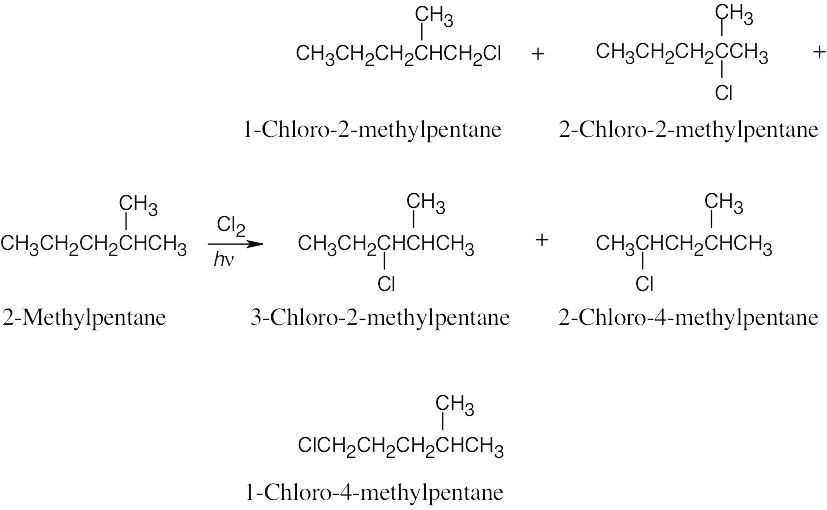
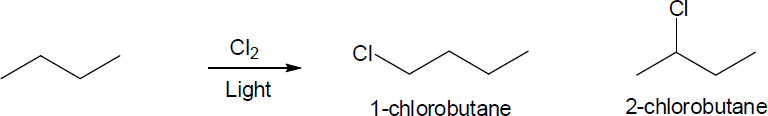
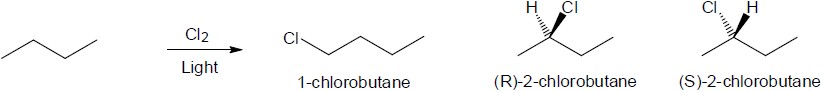
| 6.35 | Pentane has three types of hydrogen atoms, CH3CH2CH2CH2CH3 . Although monochlorination produces CH3CH2CH2CH2CH2Cl, it is not possible to avoid producing CH3CH2CH2CH(Cl)CH3 and CH3CH2CH(Cl)CH2CH3 as well. Since neopentane has only one type of hydrogen, monochlorination yields a single product. |
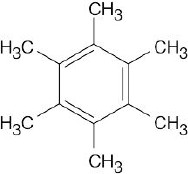
| 6.36 | The following compounds yield single monohalogenation products because each has only one kind of hydrogen atom.
|
| 6.37 | To solve these problems sequentially, replace one hydrogen on each carbon with a chlorine. If the compound has a different name, it is a different product.
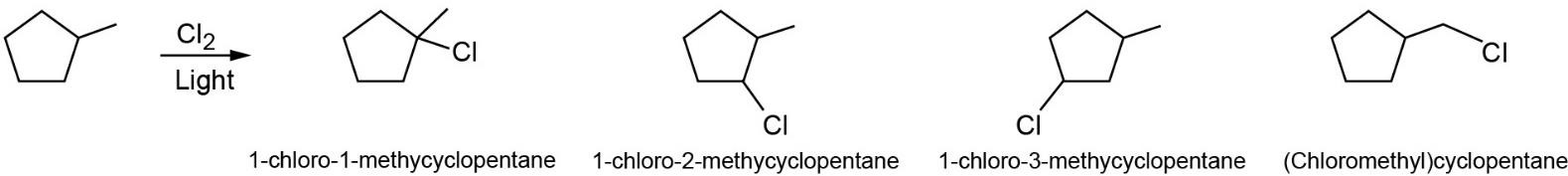
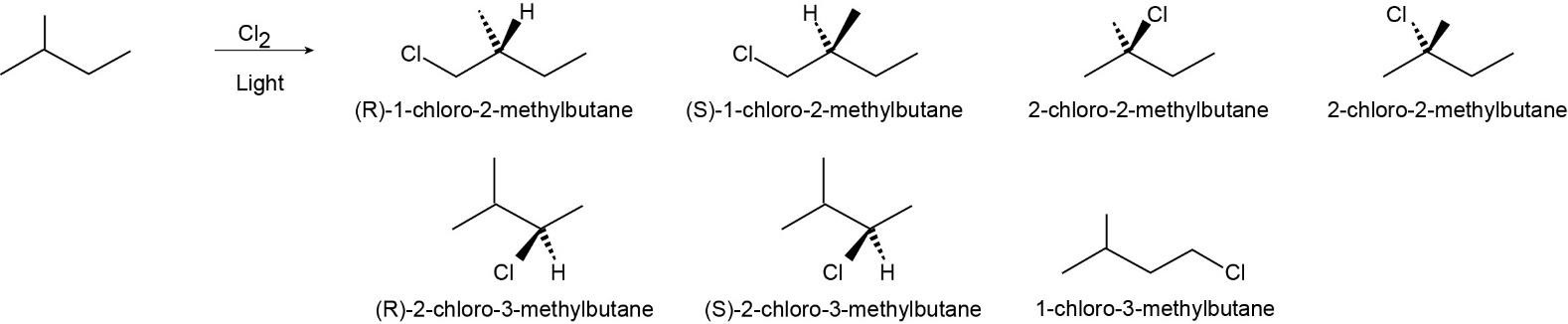
|
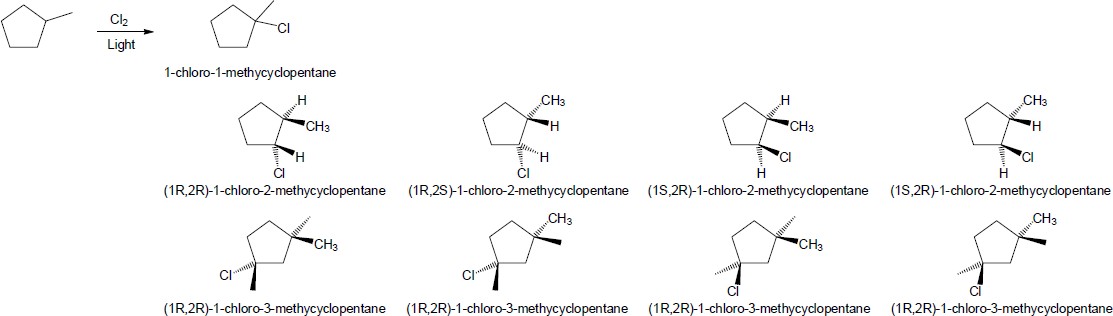
| 6.38 | To answer this question sequentially, replace each hydrogen in the molecule with a hydrogen and assign the configuration to any stereocenters. If the molecule has a different name, it is a different product.
|
| 6.39 |
|
General Problems
| 6.40 | 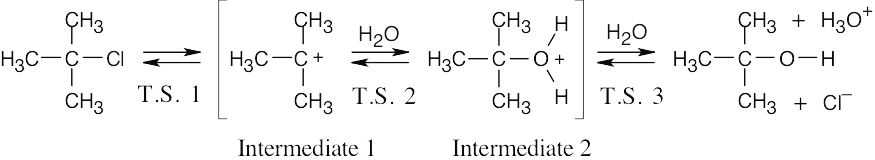 |
|
| (a) | ΔG‡ for the first step is approximately 80 kJ/mol because the reaction takes place slowly at room temperature. ΔG‡ values for the second and third steps are smaller – perhaps 60 kJ/mol for Step 2, and 40 kJ/mol for Step 3. ΔG° is approximately zero because Keq is close to 1. | |
| (b) | 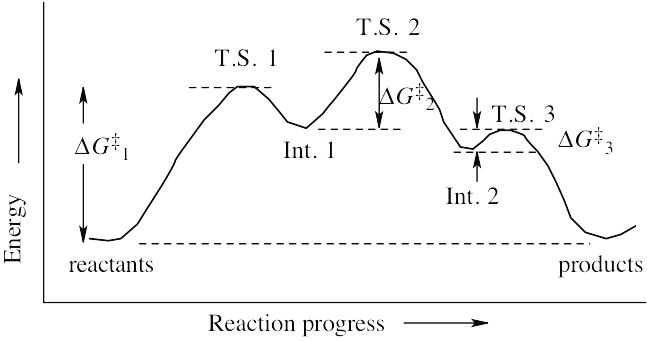 |
| 6.41 |
|
| 6.42 | ΔG° = ΔH° – TΔS°
= –75 kJ/mol – (298 K) (0.054 kJ/K·mol) = –75 kJ/mol – 16 kJ/mol = –91 kJ/mol The reaction is exothermic because ΔH° is negative, and it is exergonic because ΔG° is negative. |
| 6.43 |
|
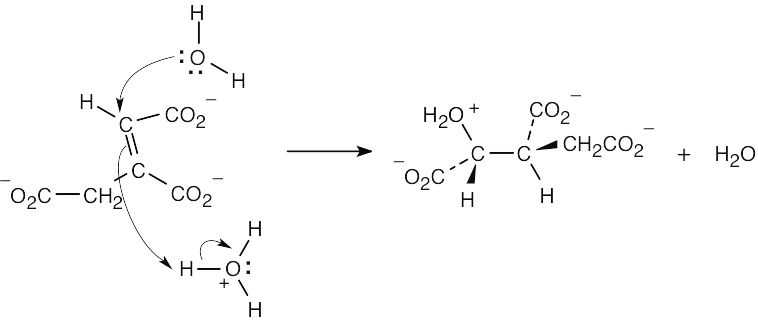
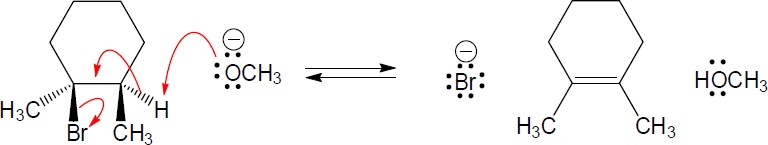
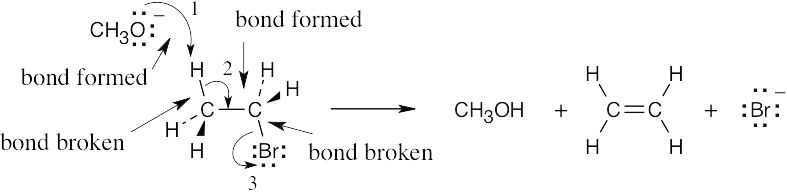
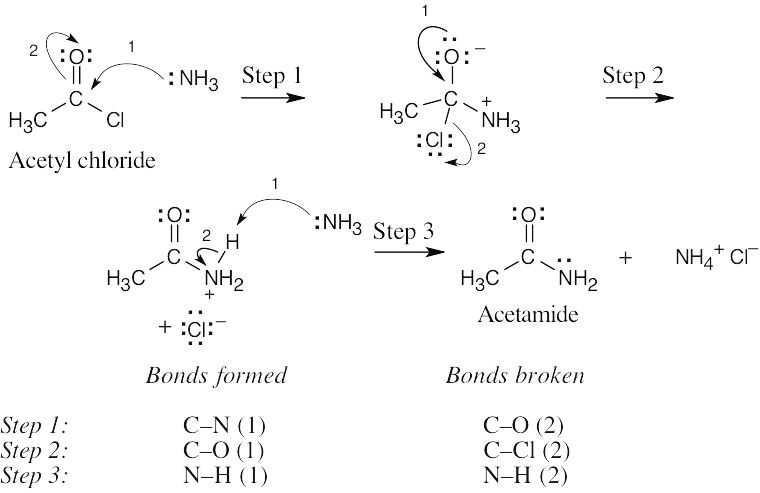
| 6.44 | Each arrow represents either the formation of a bond or the breaking of a bond. The numbers over the arrows identify the bonds broken and formed.
|
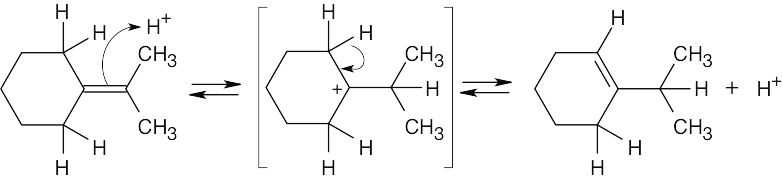
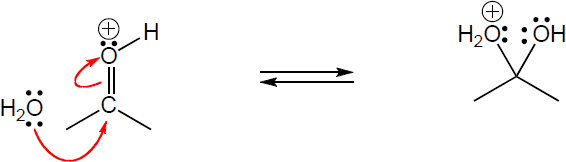
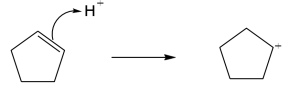
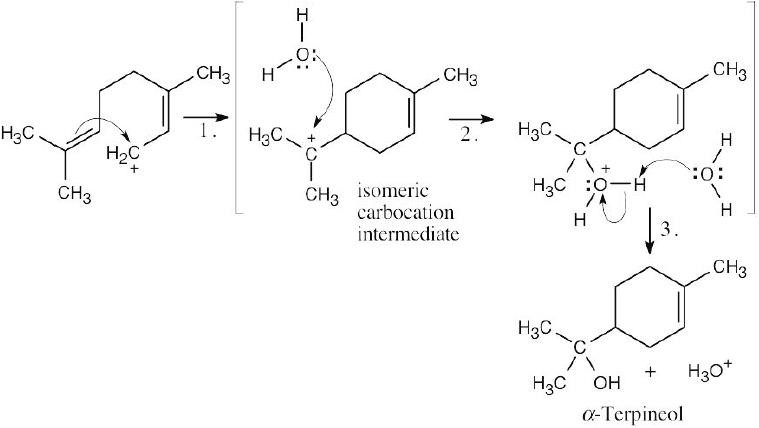
| 6.45 |
Step 1: Attack of the double bond π electrons on the carbocation to form the isomeric carbocation. Step 2: Addition of water to the intermediate carbocation. Step 3: Deprotonation. |
| 6.46 |
|
| 6.47 |
|
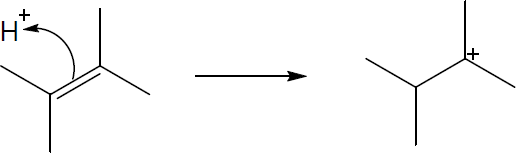
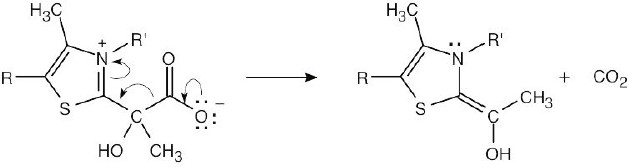
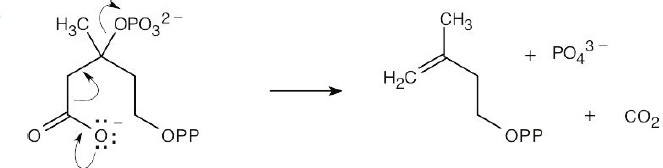
| 6.48 |
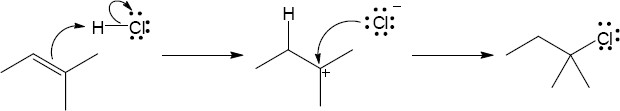
The second carbocation is more stable because more alkyl substituents are bonded to the positively charged carbon. |