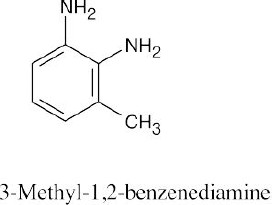
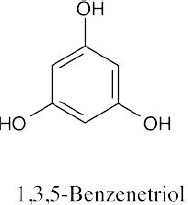
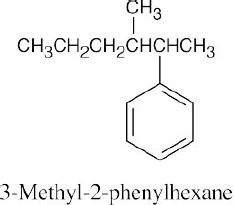
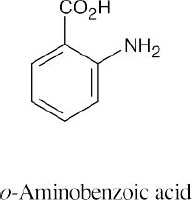
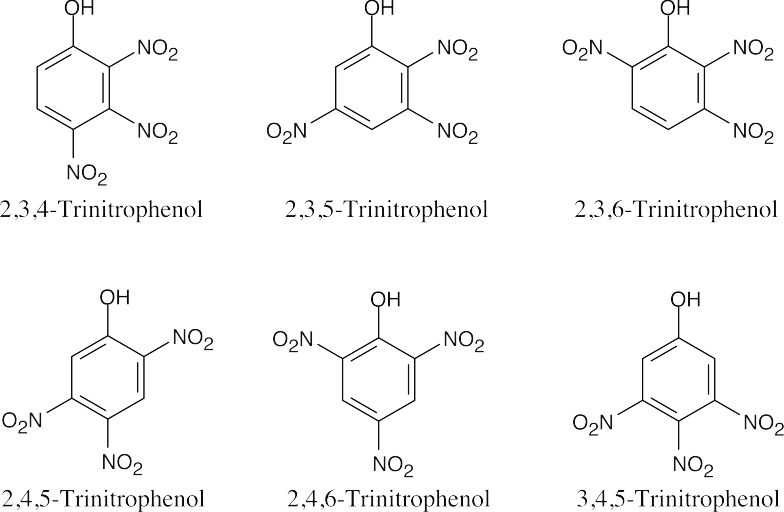
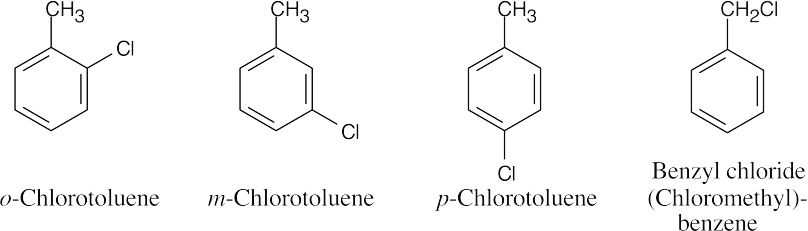
15 Chapter 15 – Benzene and Aromaticity Solutions to Problems
| 15.1 |
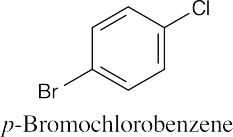
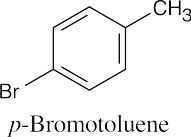
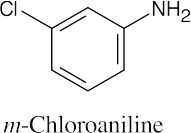
An ortho disubstituted benzene has two substituents in a 1,2 relationship. A meta disubstituted benzene has two substituents in a 1,3 relationship. A para disubstituted benzene has two substituents in a 1,4 relationship. |
|
(a) |
 |
(b) |
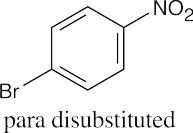 |
(c) |
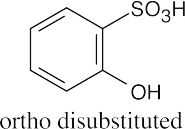 |
| 15.2 |
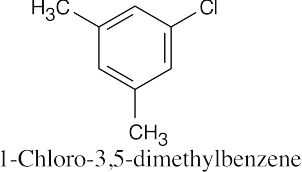
Remember to give the lowest possible numbers to substituents on trisubstituted rings. |
|
(a) |
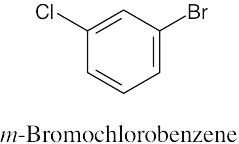 |
(b) |
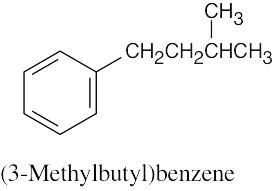 |
(c) |
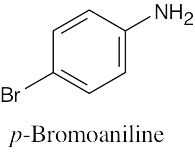 |
|
(d) |
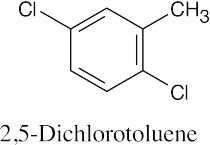 |
(e) |
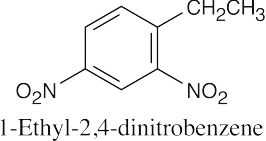 |
(f) |
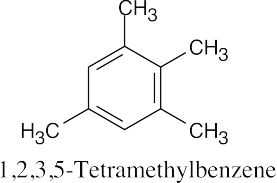 |
| 15.4 |
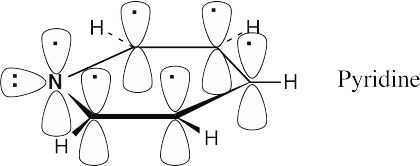
The electronic descriptions of pyridine and benzene are very similar. The pyridine ring is formed by the σ overlap of carbon and nitrogen sp2 orbitals. In addition, six p orbitals, perpendicular to the plane of the ring, hold six electrons. These six p orbitals form six π molecular orbitals that allow electrons to be delocalized over the π system of the pyridine ring. The lone pair of nitrogen electrons occupies an sp2 orbital that lies in the plane of the ring.
|
| 15.5 |
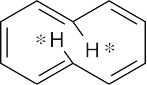
Cyclodecapentaene has 4n + 2 π electrons (n = 2), but it is not flat. If cyclodecapentaene were flat, the starred hydrogen atoms would crowd each other across the ring. To avoid this interaction, the ring system is distorted from planarity.
|
| 15.6 |
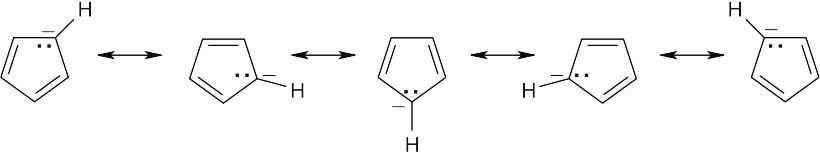
A compound that can be described by several resonance forms has a structure that can be represented by no single form. The structure of the cyclopentadienyl anion is a hybrid of all of the above structures and contains only one kind of carbon atom and one kind of hydrogen atom. All carbon–carbon bond lengths are equivalent, as are all carbon– hydrogen bonds lengths. Both the 1H NMR and 13C NMR spectra show only one absorption.
|
| 15.7 |
When cyclooctatetraene accepts two electrons, it becomes a (4n + 2) π electron aromatic ion. Cyclooctatetraenyl dianion is planar with a carbon–carbon bond angle of 135°, that of a regular octagon. |
| 15.8 |
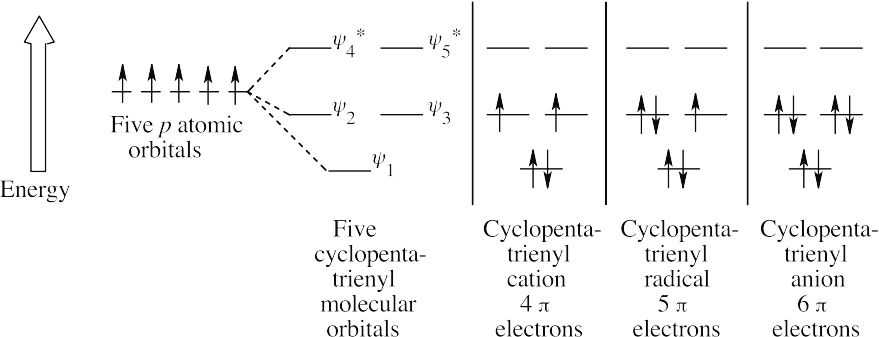
This diagram resembles Figure 15.6 but has one less antibonding orbital.
|
| 15.9 |
Furan is the oxygen analog of pyrrole. Furan is aromatic because it has 6 π electrons in a cyclic, conjugated system. Oxygen contributes two lone-pair electrons from a p orbital perpendicular to the plane of the ring.
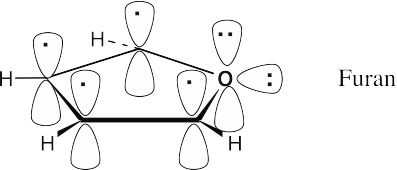
|
| 15.10 |
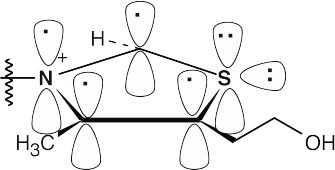
The heterocyclic thiazolium ring contains six π electrons. Each carbon contributes one electron, nitrogen contributes one electron, and sulfur contributes two electrons to the ring π system. The thiazolium ion is aromatic because it has 6 π electrons in a cyclic, planar, conjugated system.
|
| 15.11 |
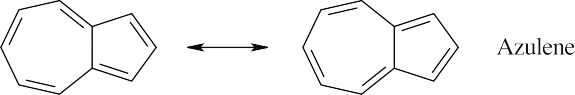
Azulene is aromatic because it has a conjugated cyclic π electron system containing ten π electrons (a Hückel number). |
| 15.12 |
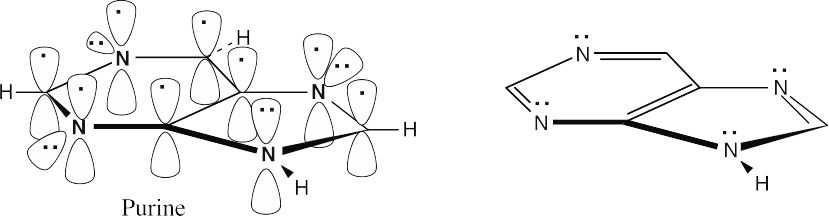
Purine is a ten-π-electron aromatic molecule. The N–H nitrogen atom in the five-membered ring donates both electrons of its lone pair to the π electron system, and each of the other three nitrogens donates one electron to the π electron system.
|
Additional Problems
Visualizing Chemistry
| 15.13 |
(a) |
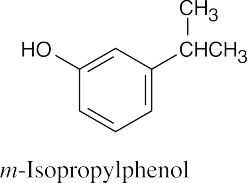 |
(b) |
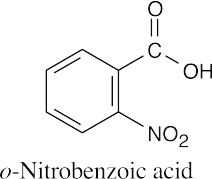 |
|
|
| 15.14 |
The all-cis cyclodecapentaene shown here is not aromatic because it is not planar. All hydrogens, however, are equivalent and show one absorption in the vinylic region of the molecule’s 1H NMR spectrum. If the molecule were planar and therefore aromatic, the absorption would appear between 6.5–8.0 δ. |
| 15.15 |
1,6-Methanonaphthalene has ten π electrons and is sufficiently planar to behave as an aromatic molecule. The perimeter hydrogens absorb in the aromatic region of the 1H NMR spectrum (6.9–7.3 δ). Interaction of the applied magnetic field with the perimeter π electrons sets up a ring current (see Section 15.7) that strongly shields the CH2 protons and causes them to absorb far upfield (–0.5 δ).
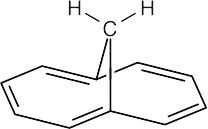
|
| 15.16 |
Three resonance forms for the carbocation of the formula C13H9 are shown below, and more can be drawn. These forms show that the positive charge of the carbocation can be stabilized in the same way as an allylic or benzylic carbocation is stabilized – by overlap with the neighboring π electrons of the ring system.
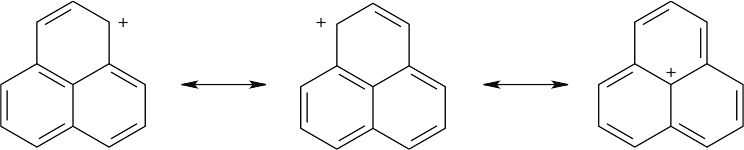
|
| 15.17 |
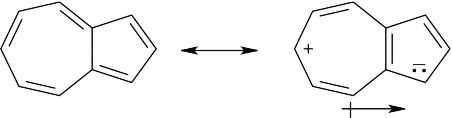
Molecules with dipole moments are polar because electron density is drawn from one part of the molecule to another. In azulene, for example, electron density is drawn from the seven-membered ring to the five-membered ring, satisfying Hückel’s rule for both rings and producing a dipole moment. The five-membered ring resembles the cyclopentadienyl anion in having six π electrons, while the seven-membered ring resembles the cycloheptatrienyl cation. The electrostatic potential map shows that the five-membered ring is more electron-rich (red) than the seven-membered ring.
|
Naming Aromatic Compounds
| 15.18 |
(a) |
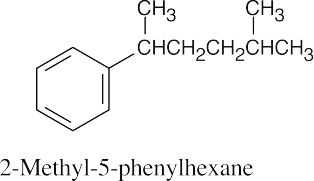 |
(b) |
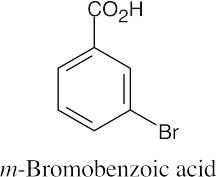 |
(c) |
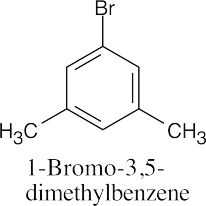 |
|
(d) |
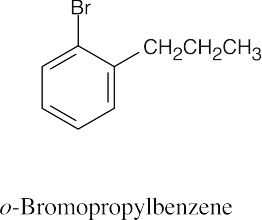 |
(e) |
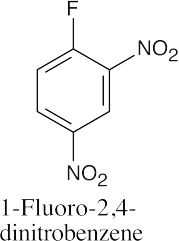 |
(f) |
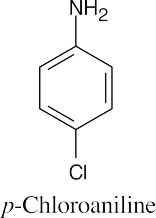 |
| 15.22 |
Six of these compounds are illustrated and named in Problem 15.20 (b).The other eight are:
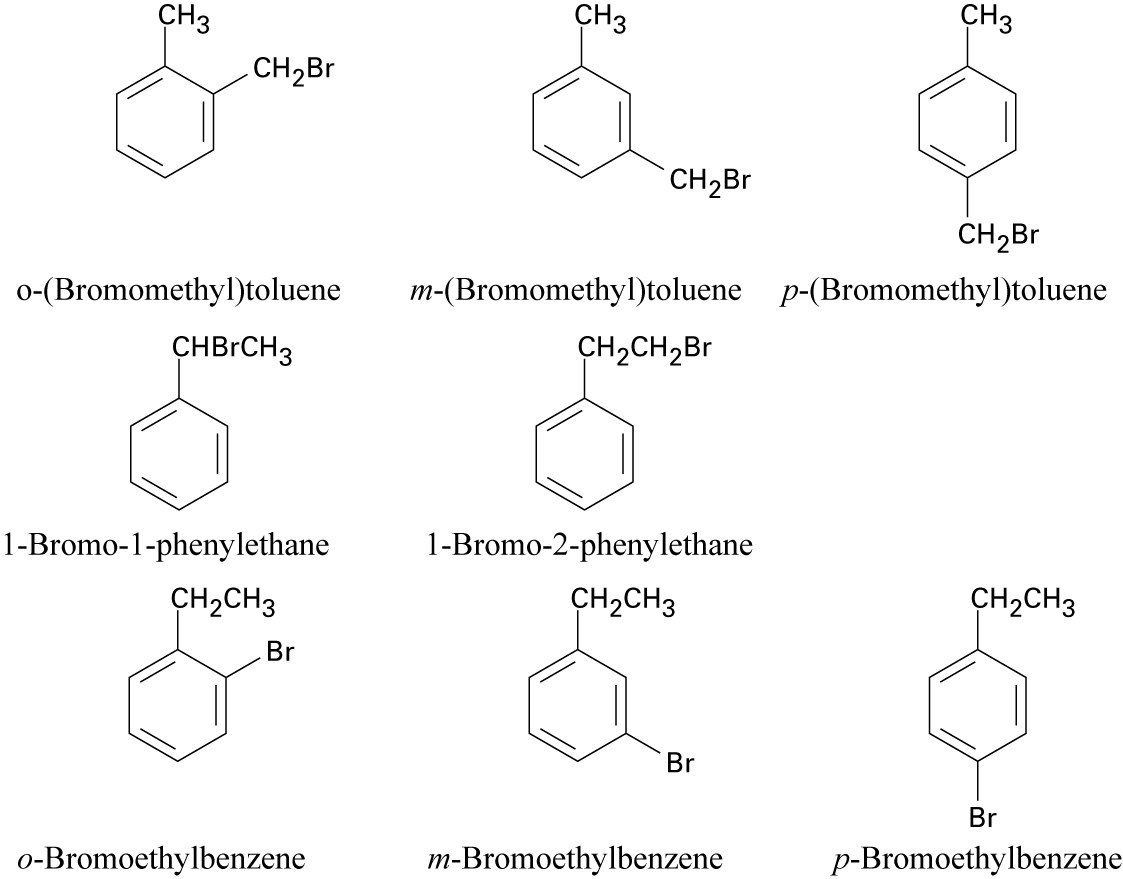
|
Structures of Organic Compounds
| 15.23 |
All compounds in this problem have four double bonds and/or rings and must be substituted benzenes, if they are to be aromatic. They may be substituted by methyl, ethyl, propyl, or butyl groups. |
|
(a) |
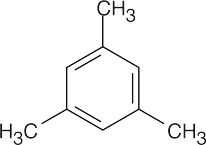 |
|
(b) |
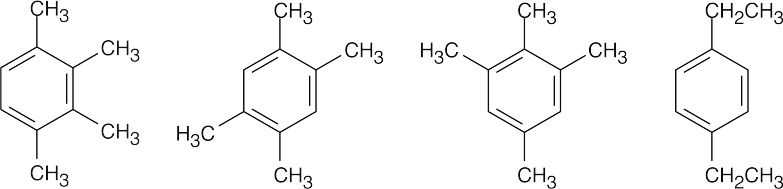 |
|
(c) |
 |
|
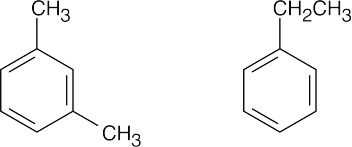
(d) |
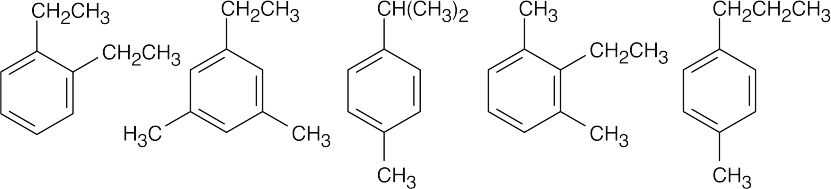 |
| 15.24 |
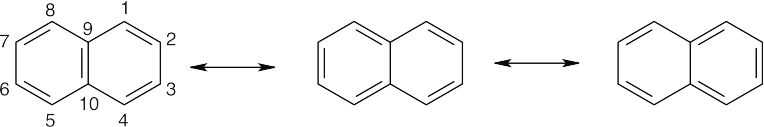
The bond between carbons 1 and 2 is represented as a double bond in two of the three resonance structures, but the bond between carbons 2 and 3 is represented as a double bond in only one resonance structure. The C1–C2 bond thus has more double-bond character in the resonance hybrid, and it is shorter than the C2–C3 bond. The C3–C4, C5–C6, and C7–C8 bonds also have more double-bond character than the remaining bonds.
|
| 15.25 |
 |
| 15.26, 15.27 |
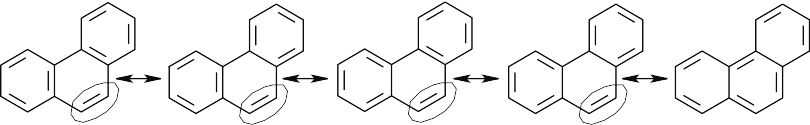
The circled bond is represented as a double bond in four of the five resonance forms of phenanthrene. This bond has more double-bond character and thus is shorter than the other carbon–carbon bonds of phenanthrene.
|
| 15.28 |
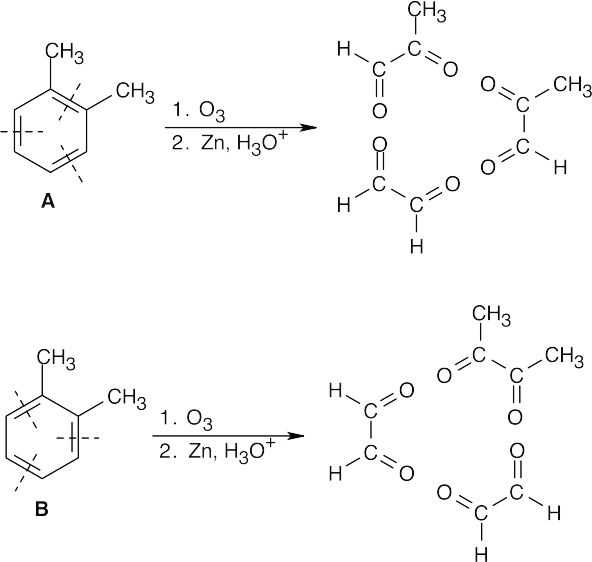
If o-xylene exists only as structure A, ozonolysis would cause cleavage at the bonds indicated and would yield two equivalents of pyruvaldehyde and one equivalent of glyoxal for each equivalent of A consumed. If o-xylene exists only as structure B, ozonolysis would yield one equivalent of 2,3-butanedione and two equivalents of glyoxal. If o–xylene exists as a resonance hybrid of A and B, the ratio of ozonolysis products would be glyoxal : pyruvaldehyde : 2,3-butanedione = 3:2:1. Since this ratio is identical to the experimentally determined ratio, we know that A and B contribute equally to the structure of o-xylene. Note that these data don’t distinguish between the resonance hybrid structure and the alternate possibility, equilibrium between two isomeric o-xylenes.
|
Aromaticity and Hückel’s Rule
| 15.29 |
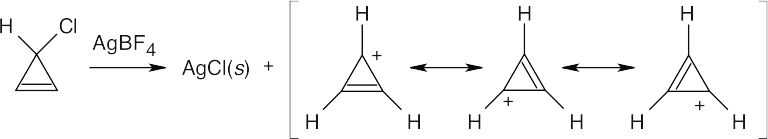
The product of the reaction of 3-chlorocyclopropene with AgBF4 is the cyclopropenyl cation C3H3+. The resonance structures of the cation indicate that all hydrogen atoms are equivalent, and the 1H NMR spectrum, which shows only one type of hydrogen atom, confirms this equivalence. The cyclopropenyl cation contains two π electrons and is aromatic according to Hückel’s rule. (Here, n = 0.) |
| 15.30 |
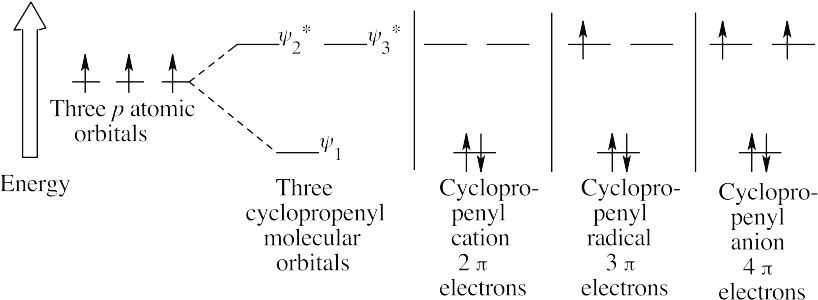
The cyclopropenyl cation is aromatic, according to Hückel’s rule.
|
| 15.31 |
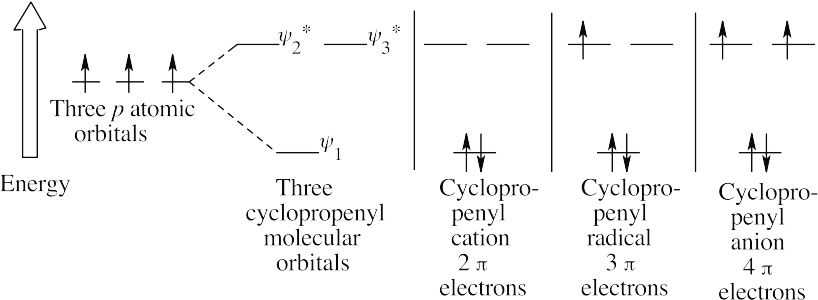
In resonance structure A, methylcyclopropenone is a cyclic conjugated compound with three π electrons in its ring. Because the electronegative oxygen attracts the π electrons of the carbon–oxygen π bond, however, a second resonance structure B can be drawn in which both carbonyl π electrons are located on oxygen, leaving only two π electrons in the ring. Since 2 is a Hückel number, the methylcyclopropenone ring is aromatic and is expected to be stable.
|
| 15.32 |
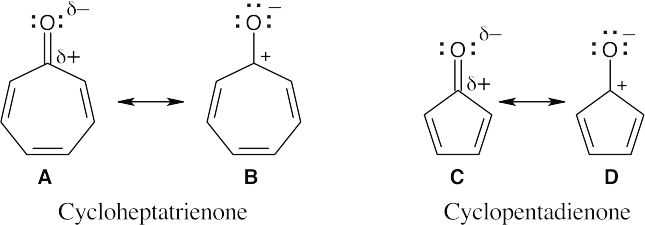
As in the previous problem, we can draw resonance forms in which both carbonyl π electrons are located on oxygen. The cycloheptatrienone ring in B contains six π electrons and is aromatic according to Hückel’s rule. The cyclopentadienone ring in D contains four π electrons and is antiaromatic. |
| 15.33 |
Check the number of electrons in the π system of each compound. The species with a Hückel (4n + 2) number of π electrons is the most stable.
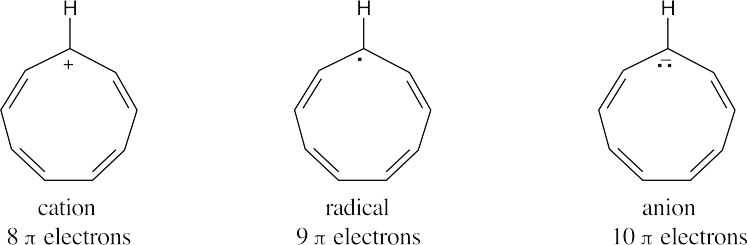
The 10 π electron anion is the most stable. |
| 15.34 |
Treat 1,3,5,7-cyclononatetraene with a strong base to remove a proton.
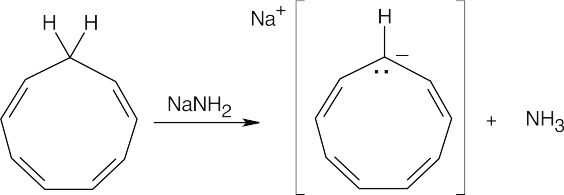
|
| 15.35 |
As with azulene, redistribution of the π electrons of calicene produces a resonance form in which both rings are aromatic and which has a dipole moment (see Problem 15.17).

|
| 15.36 |
Pentalene has eight π electrons and is antiaromatic. Pentalene dianion, however, has ten π electrons and is a stable, aromatic ion |
| 15.37 |
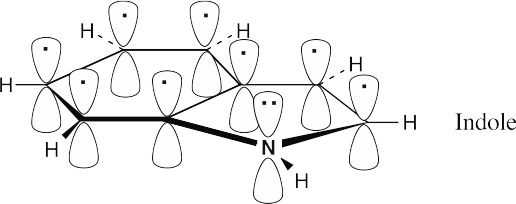
Indole, like naphthalene, has ten π electrons in two rings and is aromatic. Two π electrons come from the nitrogen atom.
|
| 15.38 |
The 1,2,4-triazole ring is aromatic because it has 6 π electrons in a cyclic, conjugated system.
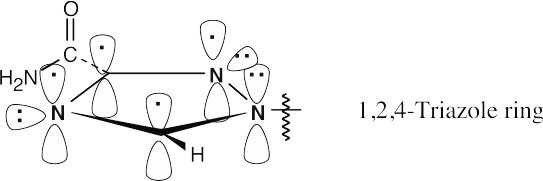
|
Spectroscopy
| 15.39 |
Compound A has four multiple bonds and/or rings. Possible structures that yield three monobromo substitution products are:
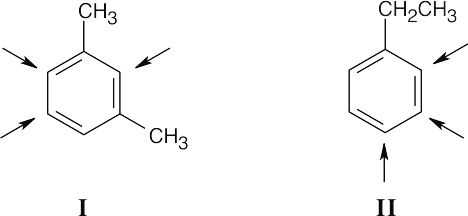
Only structure I shows a six-proton singlet at 2.30 δ, because it contains two identical benzylic methyl groups unsplit by other protons. The presence of four protons in the aromatic region of the 1H NMR spectrum confirms that I is the correct structure. |
| 15.40 |
Themolecular weight of the hydrocarbon (120) corresponds tothemolecular formula C9H12, which indicates four double bonds and/or rings. The 1H NMR singlet at 7.25 δ indicates five aromatic ring protons. The septet at 2.90 δ is due to a benzylic proton that has six neighboring protons.
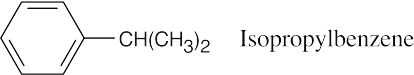
|
| 15.41 |
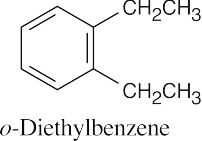
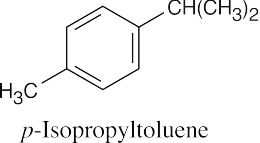
Based on degree of unsaturation, both hydrocarbons are disubstituted benzenes. The compound in (a) has two ethyl groups, and the compound in (b) has an isopropyl group and a methyl group. The IR data must be used to find the pattern of substitution.
Data in Section 15.7 show that an IR absorption of 745 cm–1 corresponds to an o-disubstituted benzene, and an absorption of 825 cm–1 corresponds to a p-disubstituted benzene. |
|
(a) |
 |
(b) |
 |
|
|
General Problems
| 15.42 |
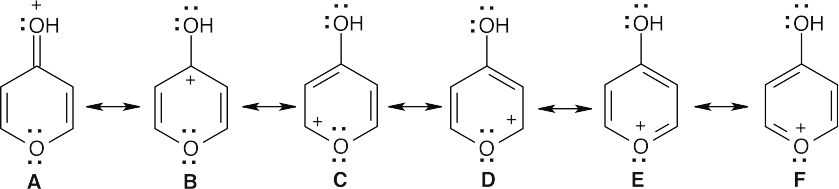
Protonation of 4-pyrone gives structure A, which has resonance forms B, C, D, E and F. In E and F, a lone pair of electrons of the ring oxygen is delocalized into the ring to produce a six π electron system, which should be aromatic according to Hückel’s rule.
|
| 15.43 |
The isoxazole ring is aromatic for the same reasons as the 1,2,4-triazole ring is aromatic (Problem 15.38).
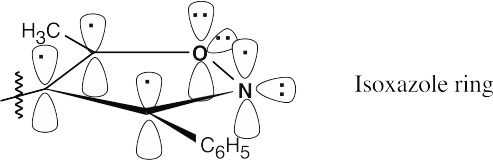
|
| 15.44 |
The second resonance form of N-phenylsydnone shows the aromaticity of the five-membered ring more clearly. In this form, the ring oxygen contributes two electrons to the ring π system, each nitrogen contributes one electron and each carbon contributes one electron (the carbonyl oxygen bears a formal negative charge). This 6 π electron, cyclic conjugated system obeys Hückel’s rule.
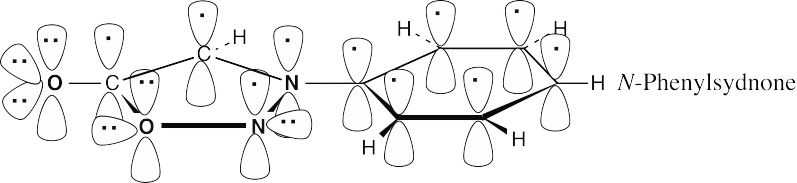
|
| 15.45 |
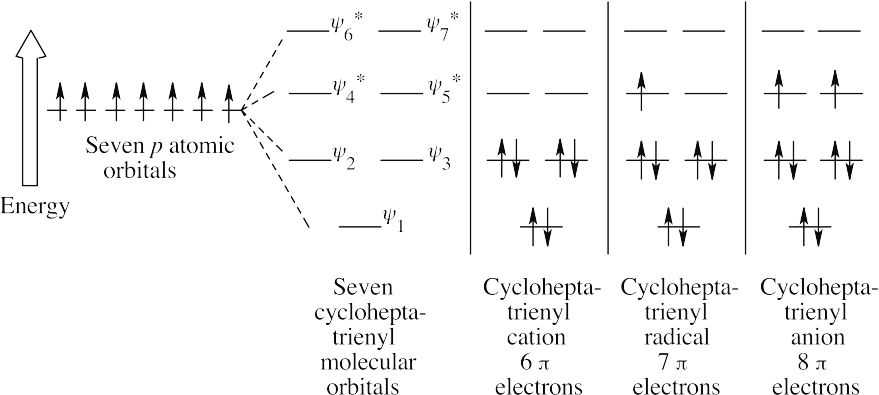
The cycloheptatrienyl cation has six π electrons (a Hückel number) and is aromatic.
|
| 15.46 |
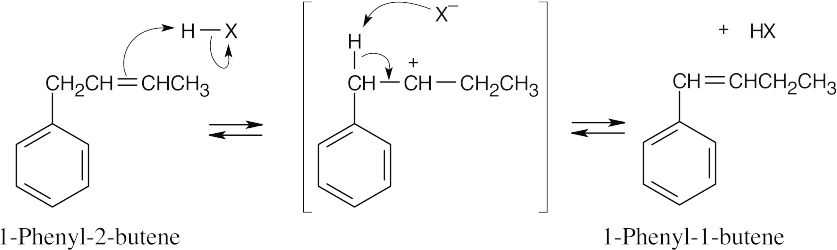
The alkene double bond is protonated to yield an intermediate carbocation, which loses a proton to give a product in which the double bond is conjugated with the aromatic ring, as shown by the increased value of λmax.
|
| 15.47 |
All of these compounds have 4 degrees of unsaturation and are substituted benzenes. The benzylic absorptions (2.3–3.0 δ) identify the hydrogens next to the aromatic ring. Remember that the data from the IR spectrum can be used to assign the substitution pattern of the ring.
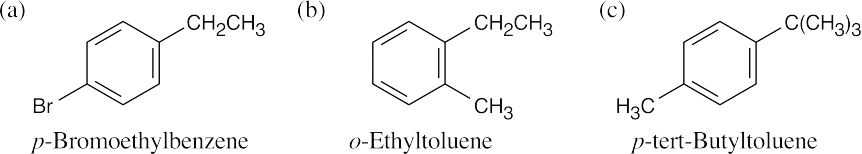
|
| 15.48 |
The compound has nine degrees of unsaturation. The 1H NMR spectrum shows that the compound is symmetrical and that the only absorptions occur in the vinylic and aromatic regions of the spectrum. The IR spectrum shows peaks due to a monosubstituted benzene ring and to R2C=CH2 (890 cm–1).
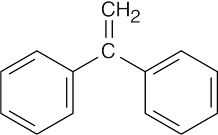
|
| 15.49 |
4-Methoxyphenylacetone
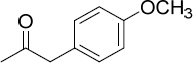
|
| 15.50 |
2,6-Dichloroaniline
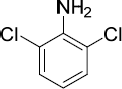
|
| 15.51 |
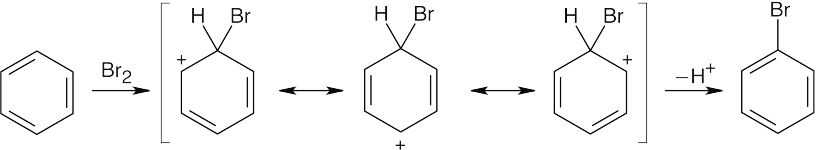 |
| 15.52 |
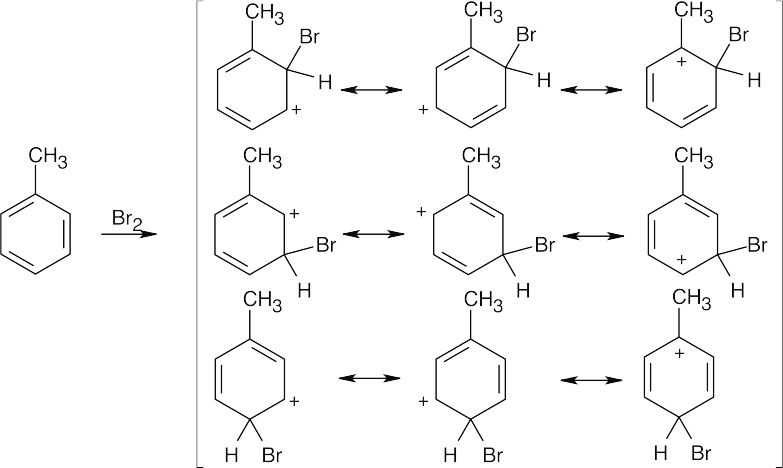
The ortho and para products predominate because the intermediate carbocation is more stabilized. The third resonance form drawn for ortho-para attack places the positive charge at the methyl-substituted carbon, which is a more stable tertiary carbocation.
|
| 15.53 |
Note: In the cyclic compounds, the charge can be placed on every atom in the ring, while in the linear compounds the charge can only reside on every other atom. |
|
(a) |
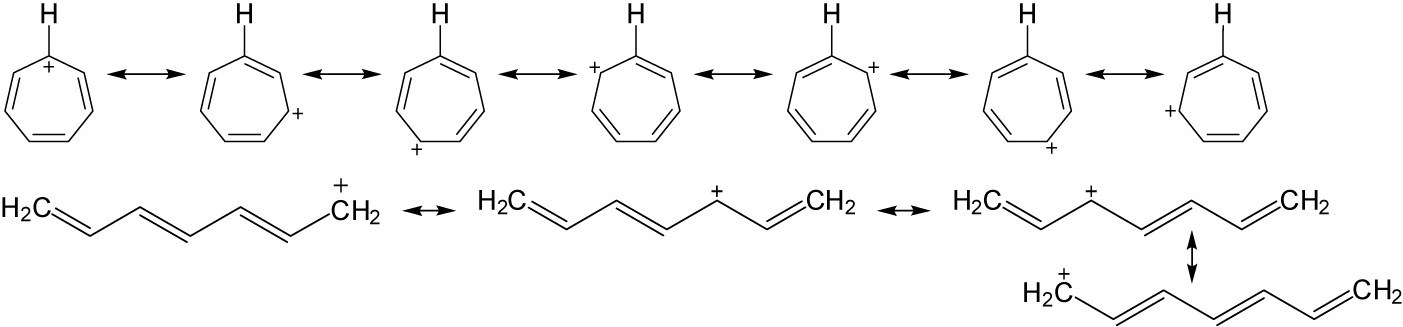 |
|
(b) |
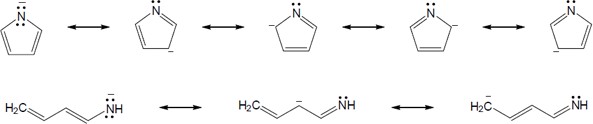 |
| 15.54 |
After the two triple bonds are connected, a new 14-membered ring is formed. This new ring is aromatic. Because of the ring current created by the new aromatic ring, the protons are shifted downfield.
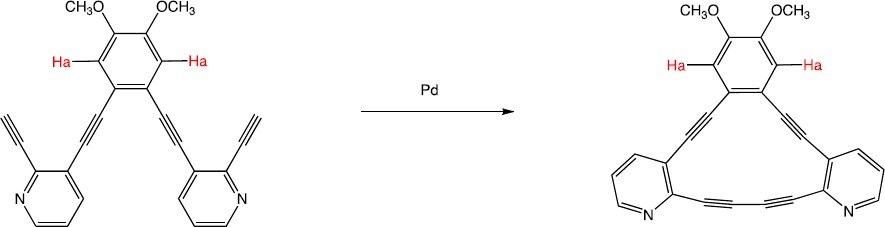
|
| 15.55 |
The tautomer has an aromatic ring, making it the more stable of the two tautomers.

|
| 15.56 |
(a) |
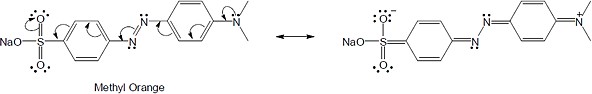 |
|
(b) |
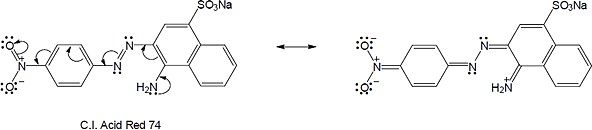 |
This file is copyright 2023, Rice University. All Rights Reserved.