25 Chapter 25 – Biomolecules: Carbohydrates Solutions to Problems
25.1
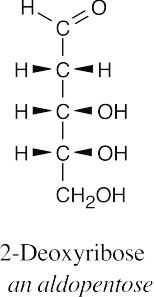 (a)(b)(c)(d)
(a)(b)(c)(d)
- Horizontal bonds of Fischer projections point out of the page, and vertical bonds point into the page.
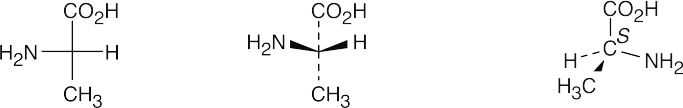 (a)
(a)
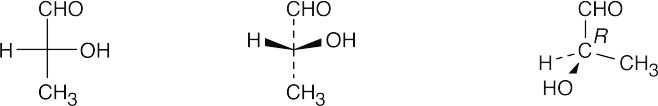 (b)
(b)
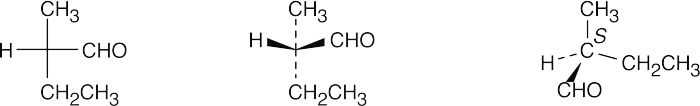 (c)
(c)
- To decide if two Fischer projections are identical, use the two allowable rotations to superimpose two groups of each projection. If the remaining groups are also superimposed after rotation, the projections represent the same enantiomer.
- Since –H is in the same position in both A and B, keep it steady, and rotate the other three groups. If, after rotation, all groups are superimposed, the two projections are identical. If only two groups are superimposed, the projections are enantiomers. Thus, A is identical to B.
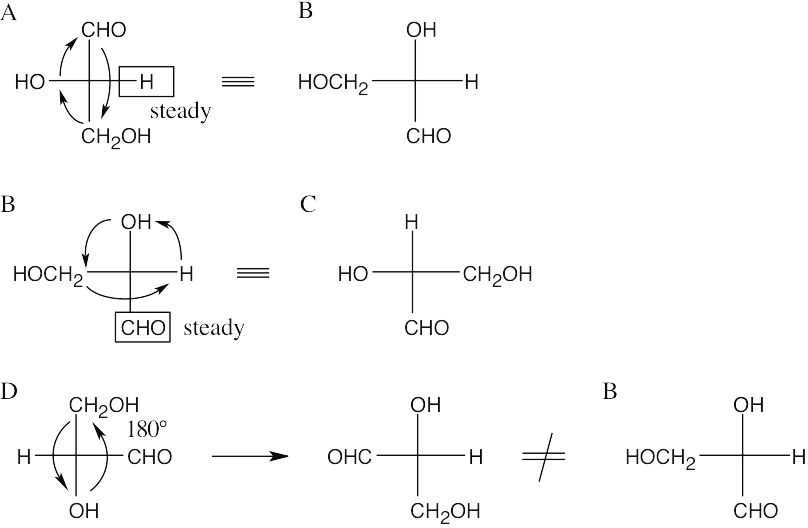
Projections A, B and C are identical, and D is their enantiomer.
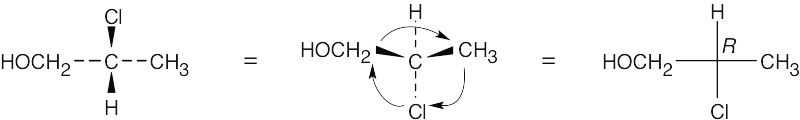
- Rotate the structure 180° around the horizontal axis to arrive at a drawing having the hydrogen at the rear. Assign the R,S configuration as usual, and draw the Fischer projection
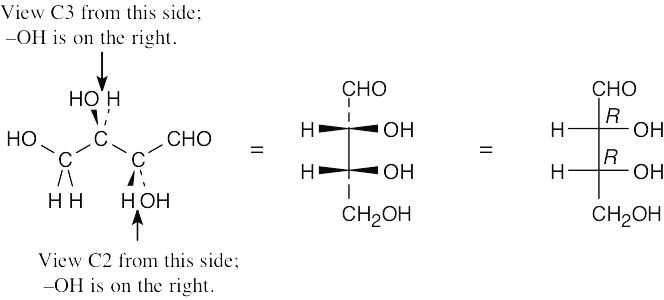
- Draw the skeleton of the Fischer projection and add the –CHO and –CH2OH groups to the top and bottom, respectively. Look at each carbon from the direction in which the –H and –OH point out of the page, and draw what you see on the Fischer projection.
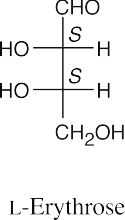
- The hydroxyl group bonded to the chiral carbon farthest from the carbonyl group points to the right in a D sugar, and points to the left in an L sugar.
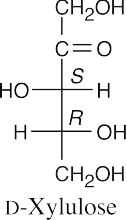 (b)(c)
(b)(c)
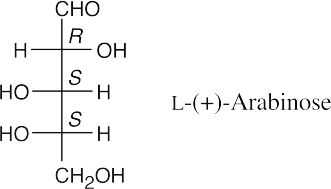
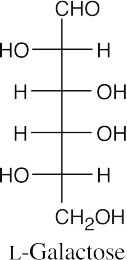
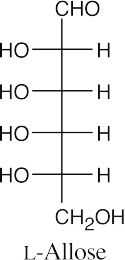 (b)(c)
(b)(c)
- An aldoheptose has 5 chirality centers. Thus, there are 25 = 32 aldoheptoses – 16 D
aldoheptoses and 16 L aldoheptoses.
- See Problem 25.5 for the method of solution.
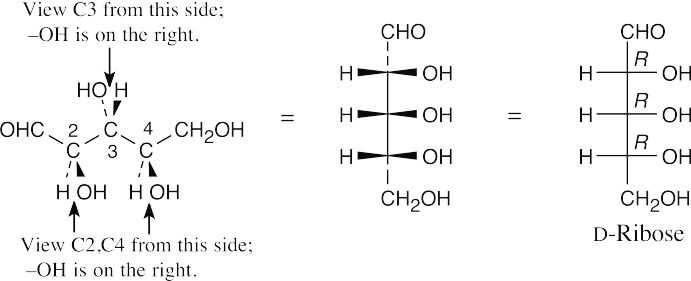
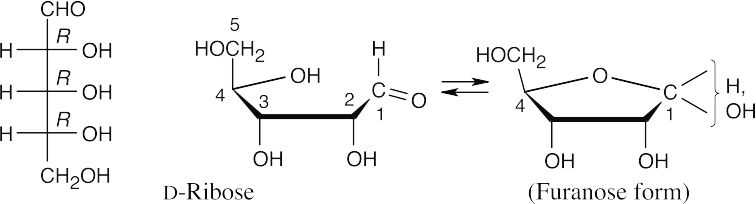
- The steps for drawing a furanose are similar to the steps for drawing a pyranose. Ring formation occurs between the –OH group at C4 and the carbonyl carbon. In transitioning from a Fischer projection to a Haworth projection, groups that were on the right side of the Fischer projection point downward in the Haworth projection.
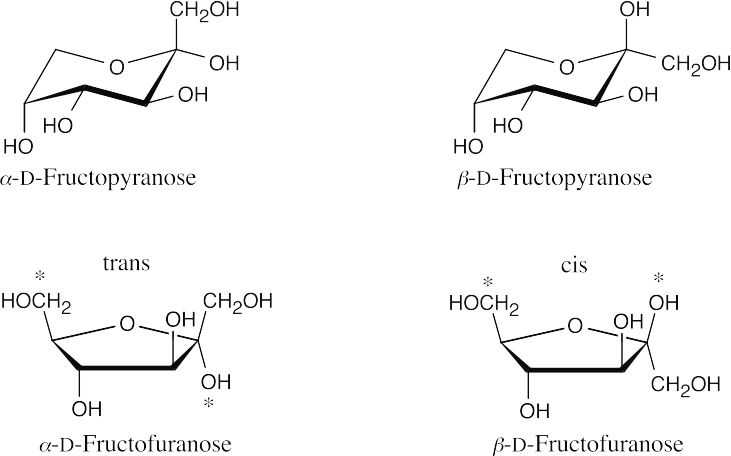
- The furanose of fructose results from ring formation between the –OH group at C5 and the ketone at C2. In the α anomer, the anomeric –OH group is trans to the C6 –CH2OH group, and in the β anomer the two groups are cis. In the pyranose form, cyclization occurs between the –OH group at C6 and the ketone. The more stable chair conformations are shown.
- There are two ways to draw these anomers: (1) Following the steps in Worked Example 25.3, draw the Fischer projection, lay it on its side, form the pyranose ring, and convert it to a chair, remembering that the anomeric –OH group is cis to the C6 group; (2) Draw β-D-glucopyranose, and exchange the hydroxyl groups that differ between glucose and the other two hexoses.
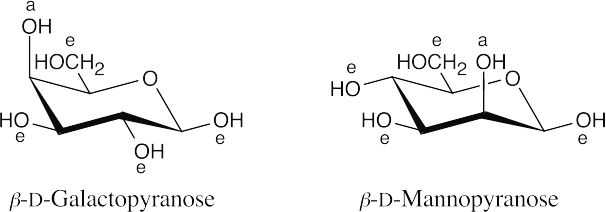
β-D-Galactopyranose and β-D-mannopyranose each have one hydroxyl group in the axial position and are therefore of similar stability.
- In the previous problem, we drew β-D-galactopyranose. In this problem, invert the configuration at each chirality center of the D enantiomer and perform a ring-flip to arrive at the structure of the L enantiomer.
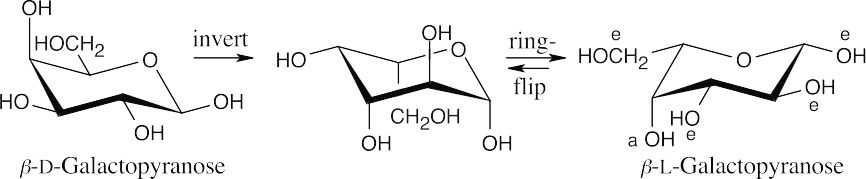
All substituents, except for the –OH at C4, are equatorial in the more stable conformation of β-L-galactopyranose.
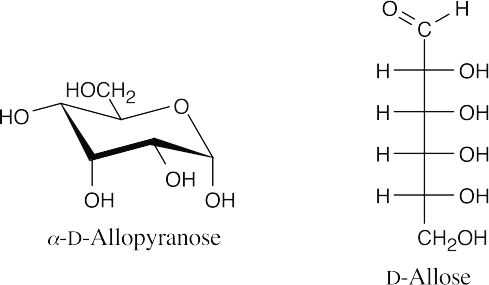
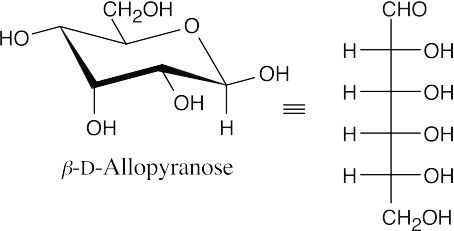
- From the model, we can see that the monosaccharide is the pyranose form of a D-hexose. It is an α-anomer because the anomeric hydroxyl group is trans to the group at C6. Comparing the model with α-D-glucopyranose, we see that all groups have the same axial/equatorial relationship, except for the hydroxyl group at C3, which is axial in the model and equatorial in α-D-glucopyranose. The monosaccharide is α-D-allopyranose. Use Figure 25.4 as a reference.
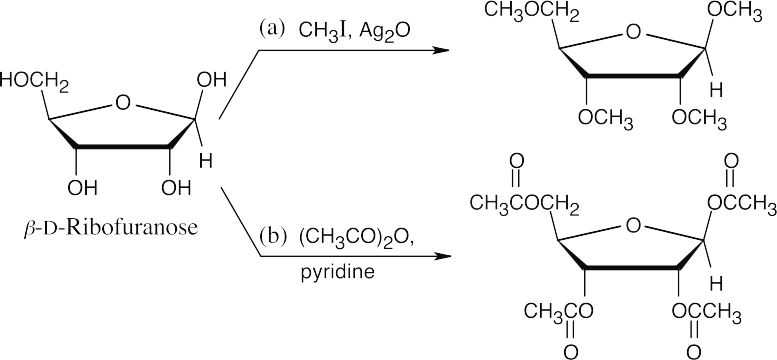
Reaction of D-galactose with NaBH4 yields an alditol that has a plane of symmetry and is a meso compound.
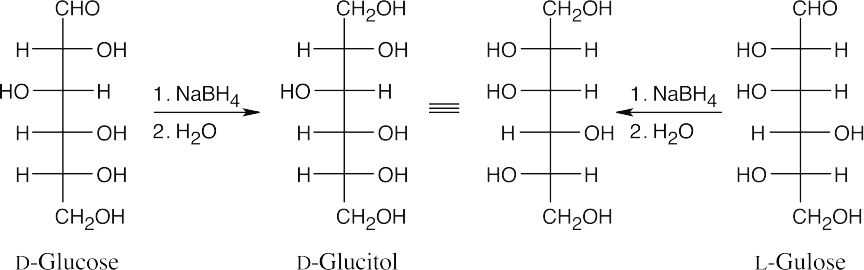
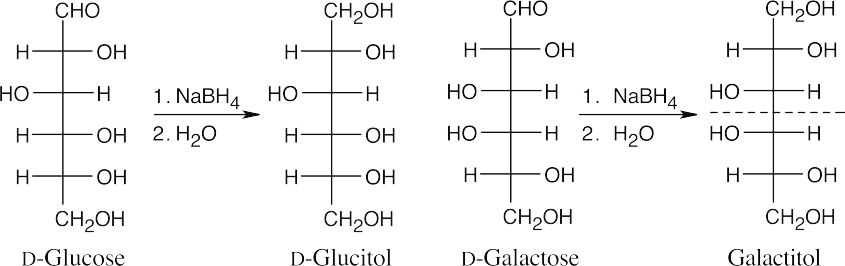
Reaction of an aldose with NaBH4 produces a polyol (alditol). Because an alditol has the same functional group at both ends, two different aldoses can yield the same alditol.
Here, L-gulose and D-glucose form the same alditol (rotate the Fischer projection of L- gulitol 180° to see the identity).
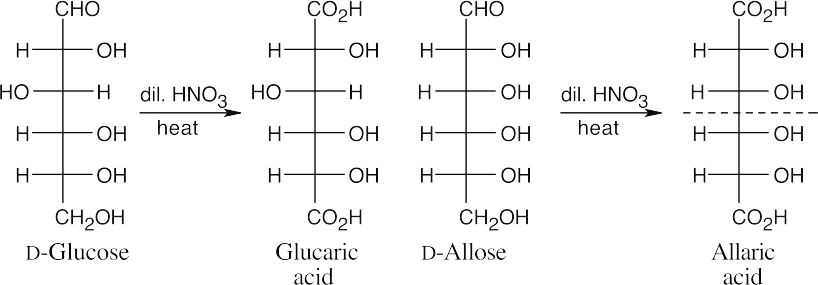
Allaric acid has a plane of symmetry and is an optically inactive meso compound. Glucaric acid has no symmetry plane.
- D-Allose and D-galactose yield meso aldaric acids. All other D-hexoses produce optically active aldaric acids on oxidation because they lack a plane of symmetry.
- The products of Kiliani–Fischer reaction of D-ribose have the same configuration at C3, C4 and C5 as D-ribose. Use Figure 25.4 as a reference.
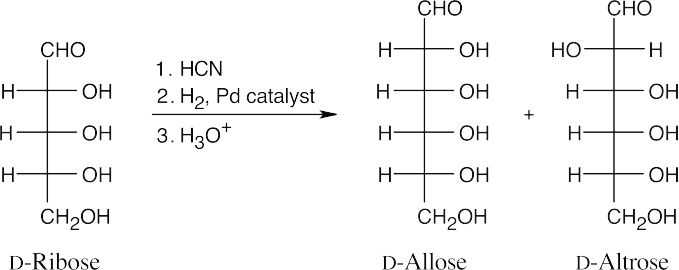
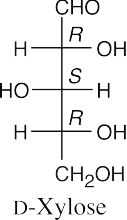
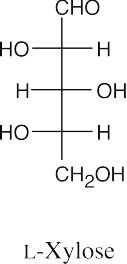
- The aldopentose, L-xylose has the same configuration as the configuration at C3, C4 and C5 of L-idose and L-gulose. Use Figure 25.4 as a reference.
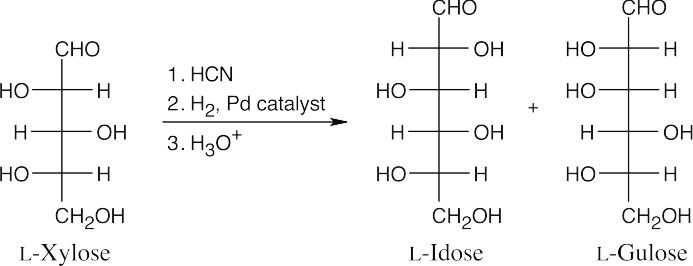
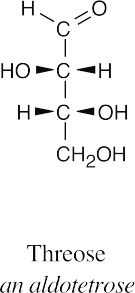
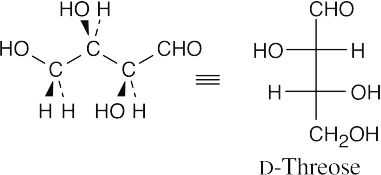
- The aldopentoses have the same configurations at C3 and C4 as D-threose.
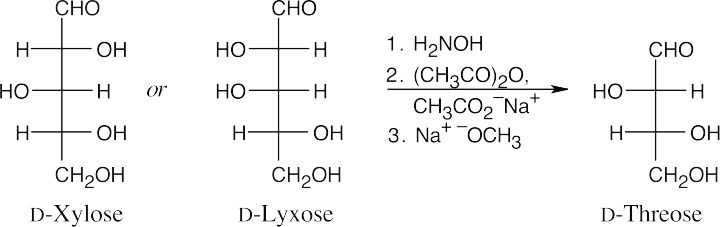
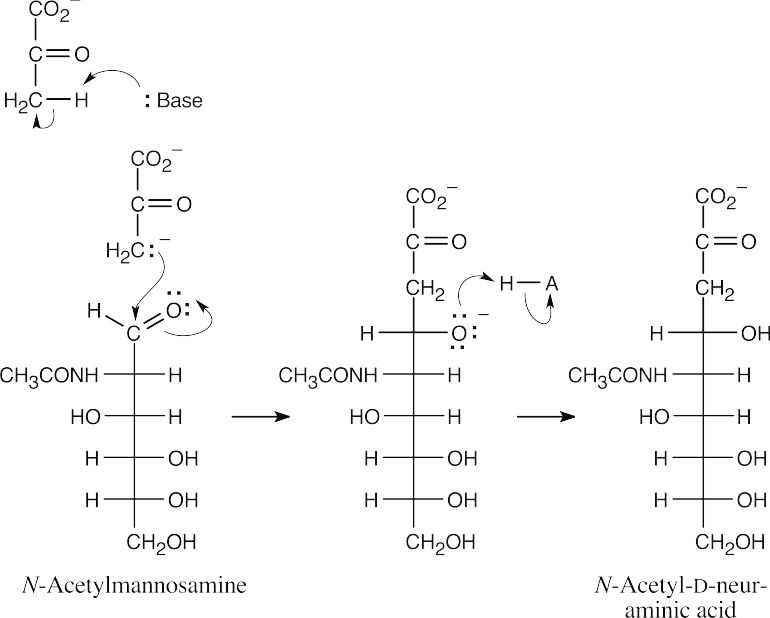
Additional Problems
Visualizing Chemistry
- (a)Convert the model to a Fischer projection, remembering that the aldehyde group is on top, pointing into the page, and that the groups bonded to the carbons below point out of the page. The model represents a D-aldose because the –OH group at the chiral carbon farthest from the aldehyde points to the right.
- Break the hemiacetal bond and uncoil the aldohexose. Notice that all hydroxyl groups point to the right in the Fischer projection. The model represents the β anomer of D-allopyranose.
- The hints in the previous problem also apply here. Molecular models are helpful.
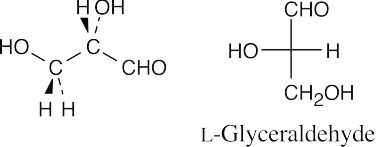
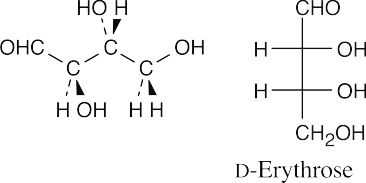 (b)
(b)
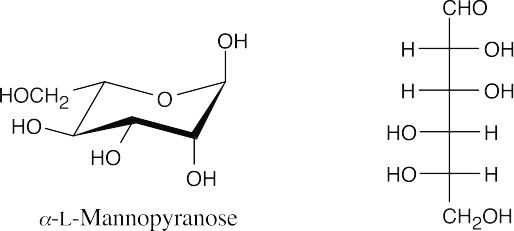
- The structure represents an α anomer because the anomeric –OH group and the –CH2OH group are trans. The compound is α-L-mannopyranose because the –OH group at C2 is the only non-anomeric axial hydroxyl group.
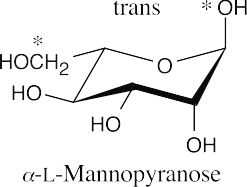
25.29 (a)
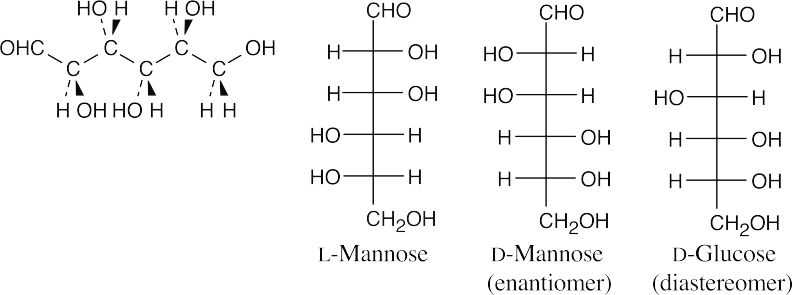
- The model represents an L-aldohexose because the hydroxyl group on the chiral carbon farthest from the aldehyde group points to the left.
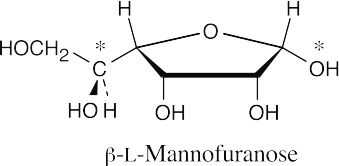
- This is tricky! The furanose ring of an aldohexose is formed by connecting the –OH group at C4 to the aldehyde carbon. The best way to draw the anomer is to lie
L-mannose on its side and form the ring. All substituents point down in the furanose, and the anomeric –OH and the –CH(OH)CH2OH group are cis.
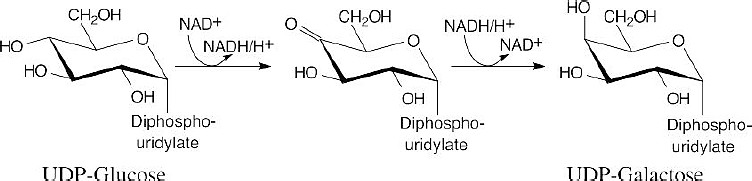 Mechanism Problems 25.30
Mechanism Problems 25.30
Oxidation of the C4 hydroxyl group by NAD+ forms a ketone plus NADH, and reduction of the ketone by NADH yields UDP-galactose. The result is an epimerization at carbon 4 of the pyranose ring.
25.31
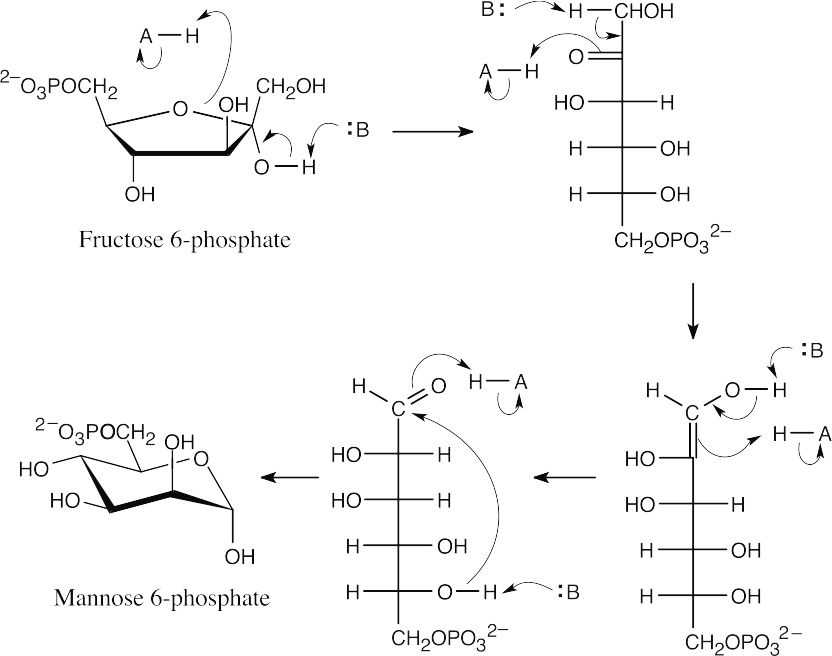
All of these reactions are acid/base catalyzed enolizations or hemiacetal openings/formations.
25.32
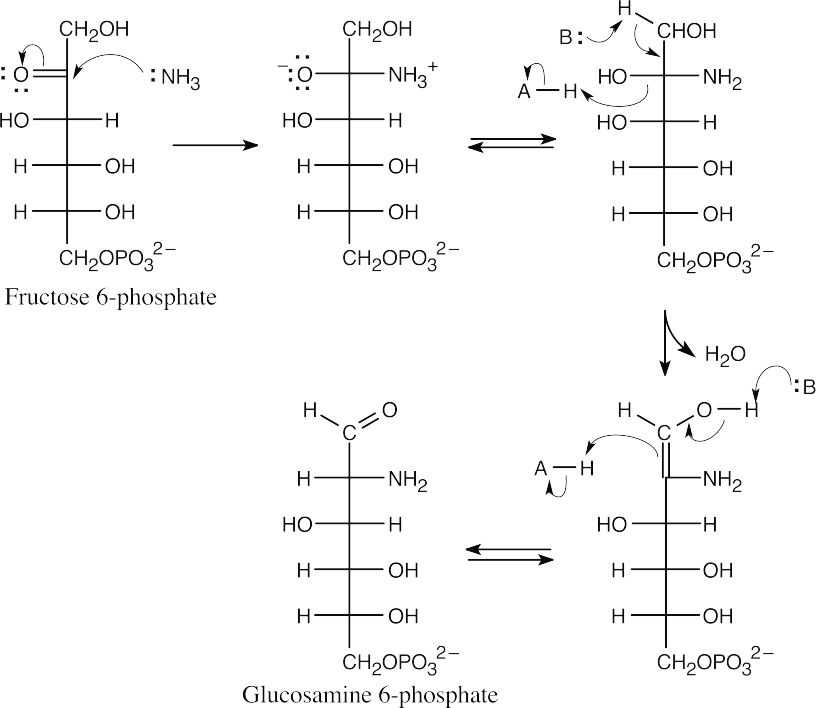
Nucleophilic acyl substitution and tautomerization lead to the formation of glucosamine 6-phosphate from fructose 6-phosphate. The mechanism of opening of the fructofuranose ring was shown in the previous problem.
- Glucopyranose is in equilibrium with glucofuranose.
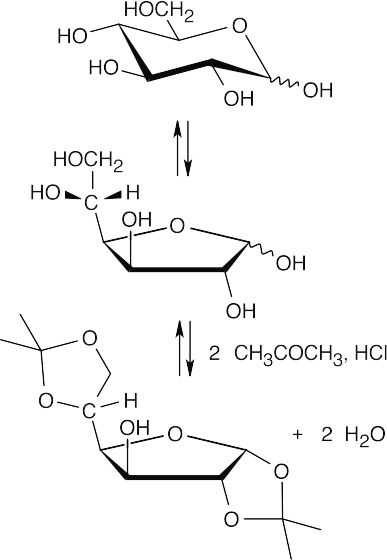
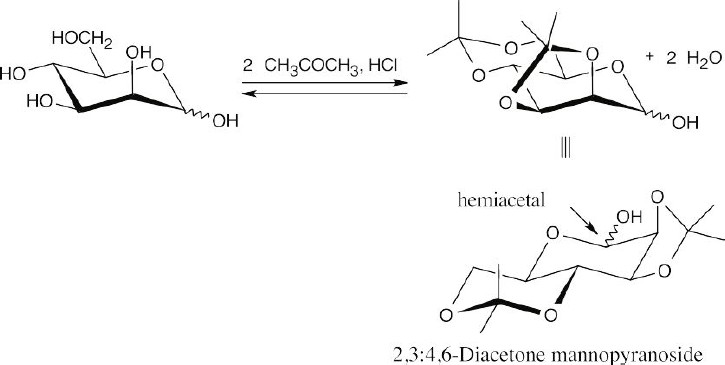
Reaction with two equivalents of acetone occurs by the mechanism we learned for acetal formation (Sec 19.10).
A five-membered acetal ring forms much more readily when the hydroxyl groups are cis to one another. In glucofuranose, the C3 hydroxyl group is trans to the C2 hydroxyl group, and acetal formation occurs between acetone and the C1 and C2 hydroxyls of glucofuranose. Since the C1 hydroxyl group is part of the acetone acetal, the furanose is no longer in equilibrium with the free aldehyde, and the diacetone derivative is not a reducing sugar.
- Cleavage of fructose 1,6-bisphosphate occurs by a retro aldol reaction.
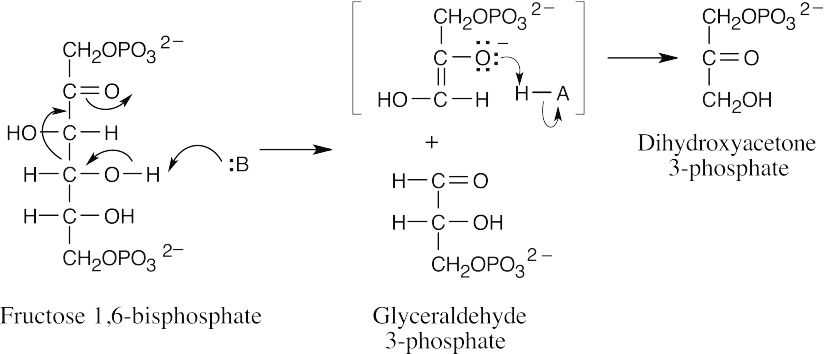
- (a)Oxidation by NADP+, elimination, and conjugate reduction by NADPH give the observed product. Notice that there is no net consumption of NADP+. The mechanism of NADP+ oxidations and reductions has been shown many times in this book and also appears in part (c).
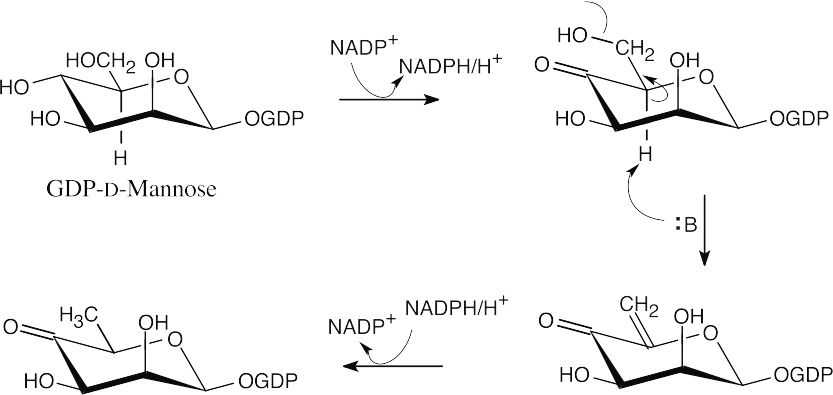
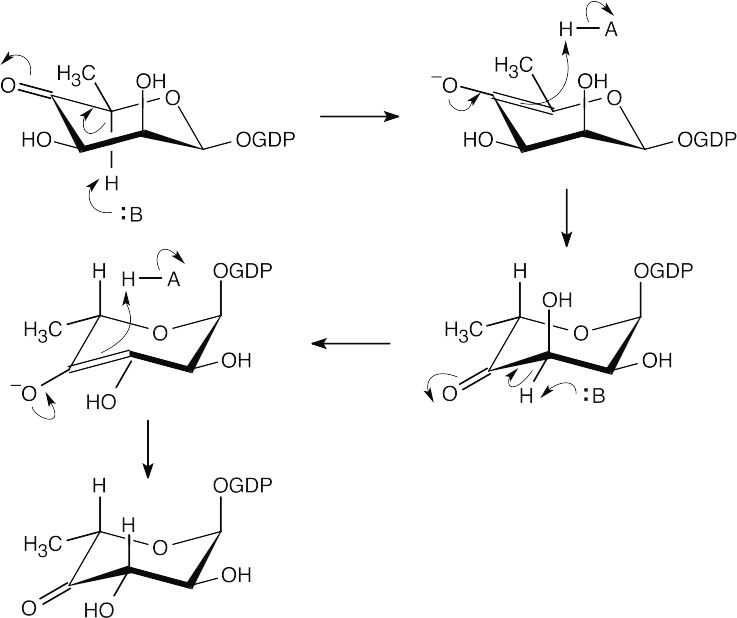
- Two epimerizations, both α to the carbonyl group, cause a change in stereochemistry.
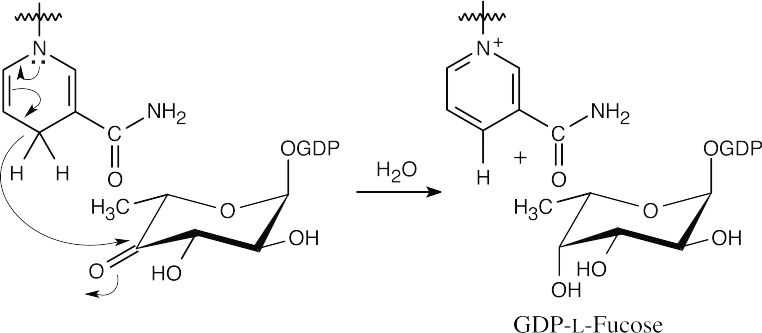
- Reduction at C4 by NADPH forms GDP-L-fucose.
Carbohydrate Structure
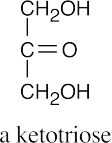
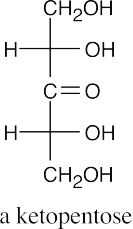
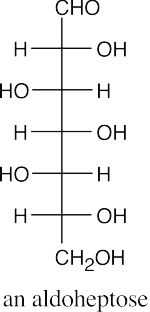 (b)(c)
(b)(c)
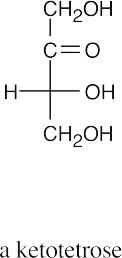
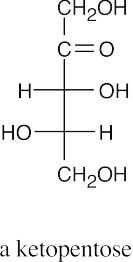
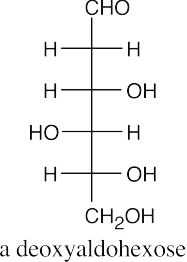
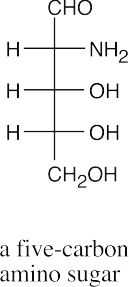 (a)(b)(c)(d)
(a)(b)(c)(d)
- D-Ribose and L-xylose are diastereomers and differ in all physical properties (or if they have identical physical properties in any category, it is a coincidence).
25.39, 25.40
Ascorbic acid has an L configuration because the hydroxyl group at the lowest chirality center points to the left.
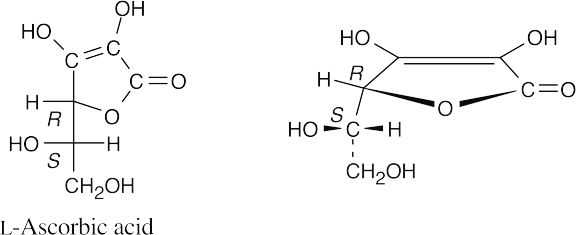
25.41
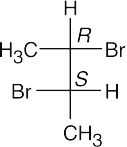
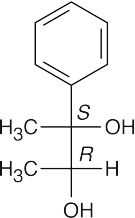
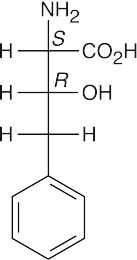 (b)(c)
(b)(c)
25.42
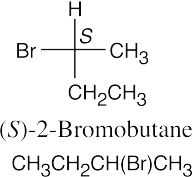
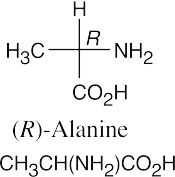 (b)
(b)
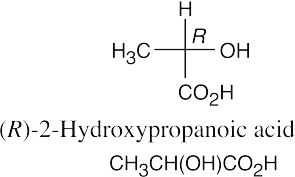
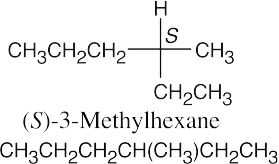 (c)(d)
(c)(d)
25.43
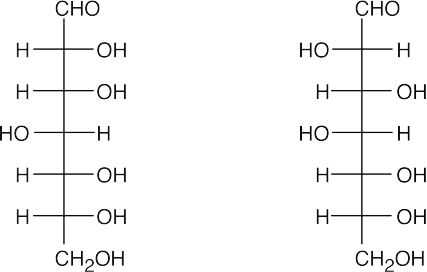
25.44
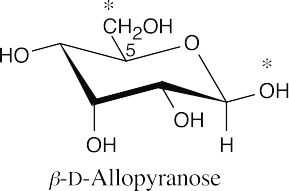
This structure is a pyranose (6-membered ring) and is a β anomer (the C1 hydroxyl group and the –CH2OH groups are cis). It is a D sugar because the -O- at C5 is on the right in the uncoiled form.
25.45
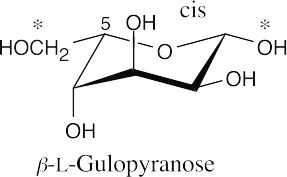
This sugar is a β-pyranose. It is an L sugar because the -O- at C5 points to the left in the uncoiled form. It’s also possible to recognize this as an L sugar by the fact that the configuration at C5 is S.
25.46
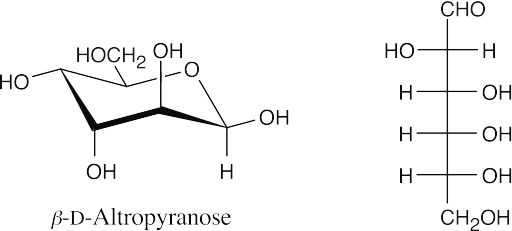 (a)
(a)
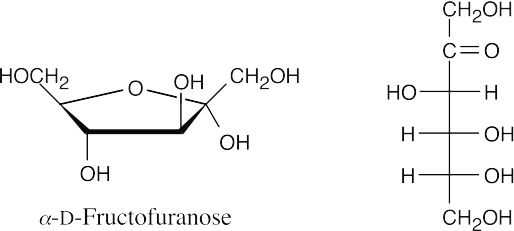 (b)
(b)
 (c)
(c)
25.47
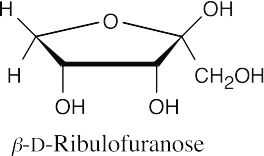
25.48
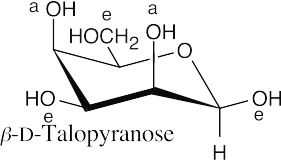
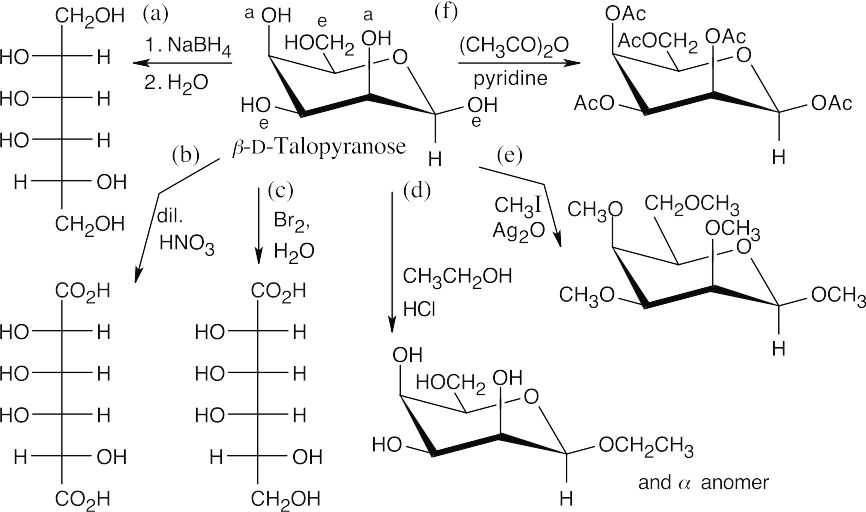 Carbohydrate Reactions 25.49
Carbohydrate Reactions 25.49
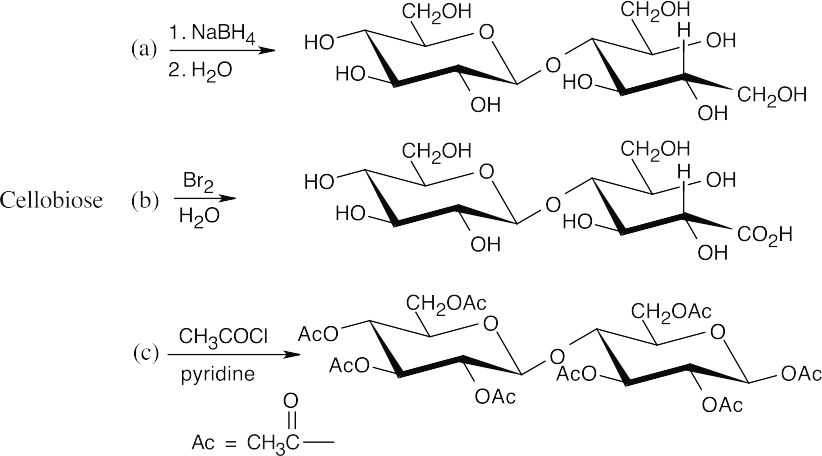
In (f), –Ac represents an acetate.
25.50-25.52 Four D-2-ketohexoses are possible.
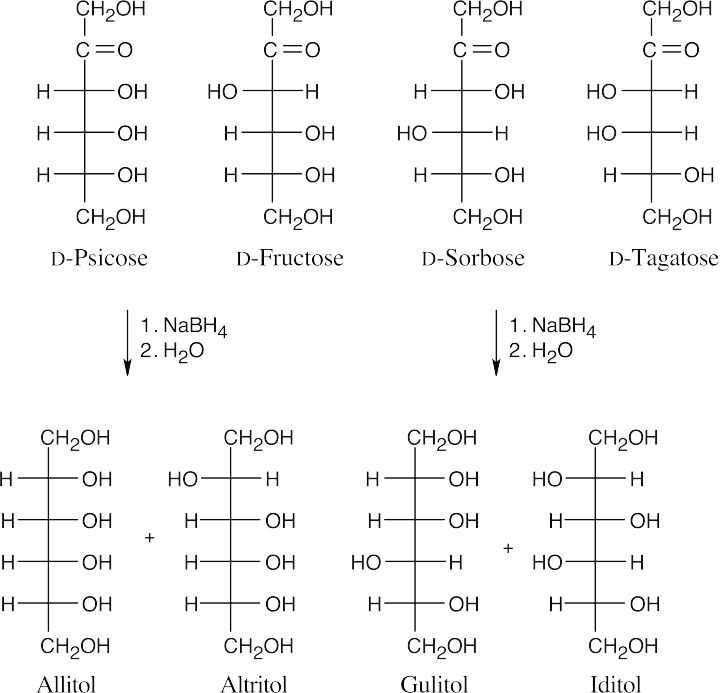
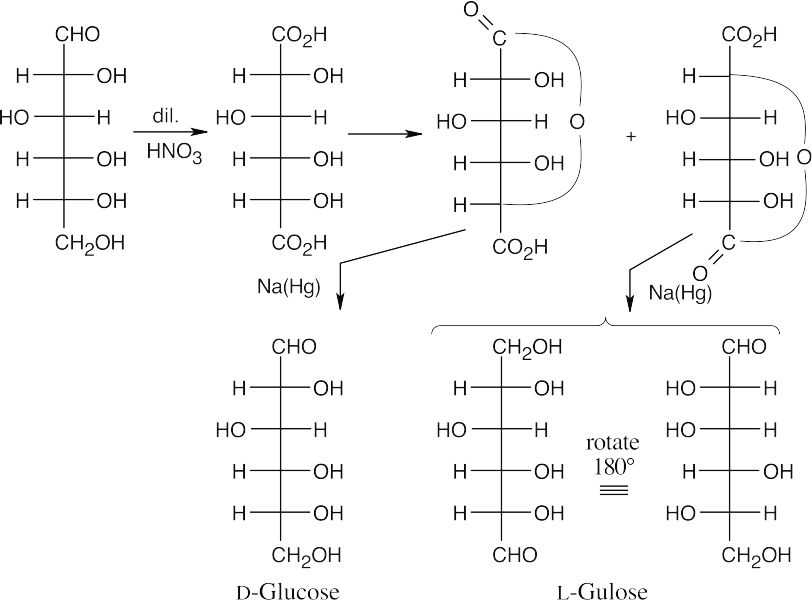
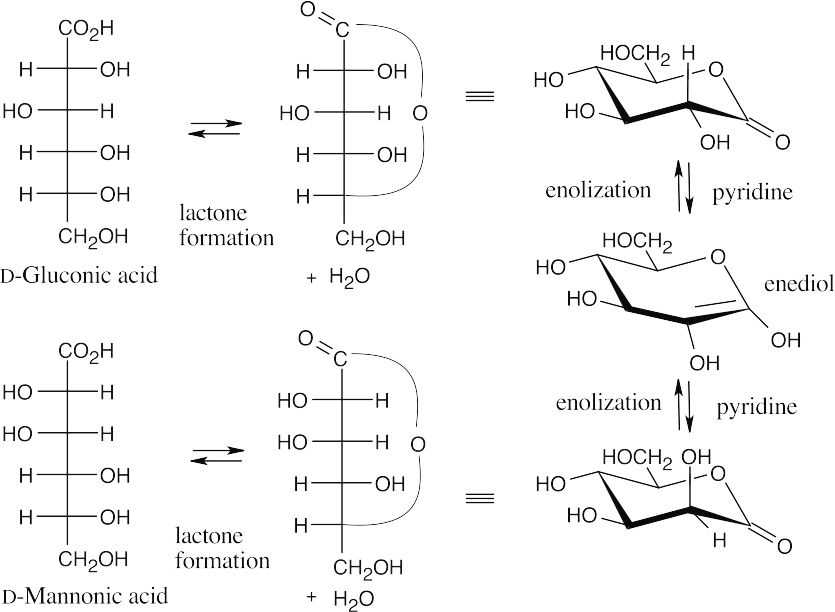
- The two lactones are formed between a carboxylic acid and a hydroxyl group 4 carbons away. When the lactones are reduced with sodium amalgam, the resulting hexoses have an aldehyde at one end and a hydroxyl group at the other end.
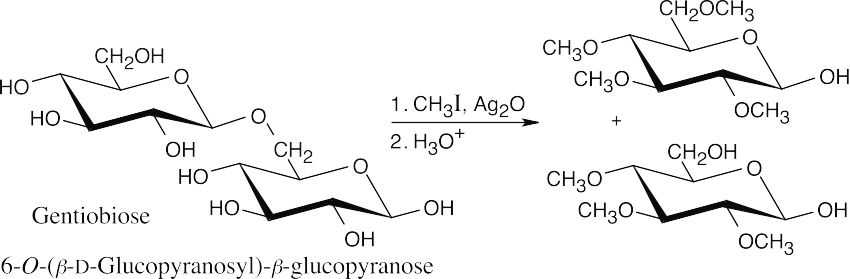
- The hard part of this problem is determining where the glycosidic bond occurs on the second glucopyranose ring. Treatment with iodomethane, followed by hydrolysis, yields a tetra-O-methyl glucopyranose and a tri-O-methyl glucopyranose. The oxygen in the
tri-O-methylated ring that is not part of the hemiacetal group and is not methylated is the oxygen that forms the acetal bond. In this problem, the C6 oxygen forms the glycosidic link.
 General Problems 25.55
General Problems 25.55
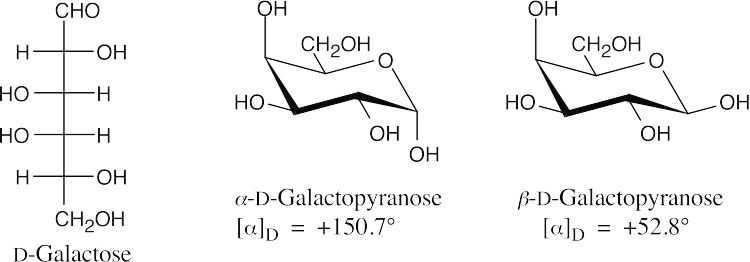
Let x be the percent of D-galactose present as the α anomer and y be the percent of
D-galactose present as the β anomer.
150.7°x + 52.8°y = 80.2°x + y = 1; y = 1 – x
150.7°x + 52.8°(1–x) = 80.2°
97.9°x = 27.4°
x = 0.280
y = 0.720
28.0% of D-galactose is present as the α anomer, and 72.0% is present as the β anomer.
25.56
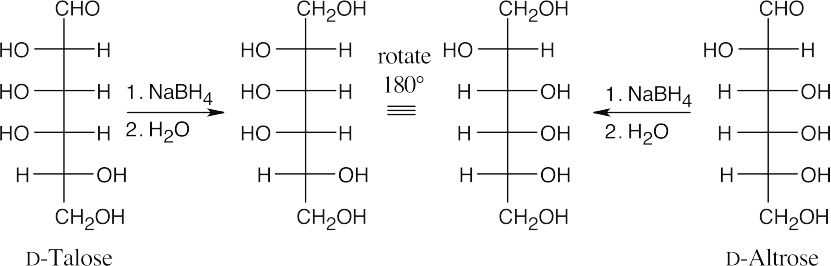
25.57
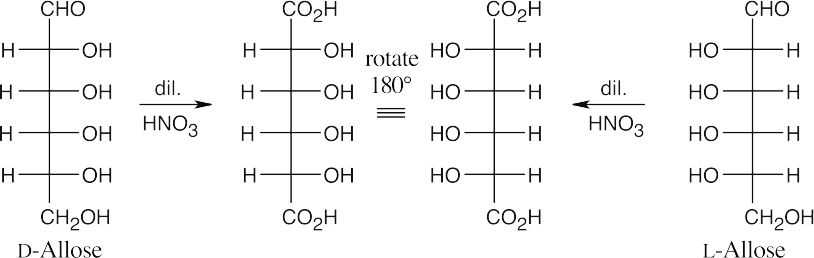
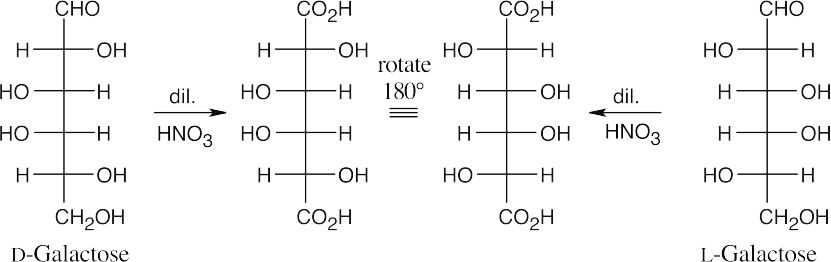
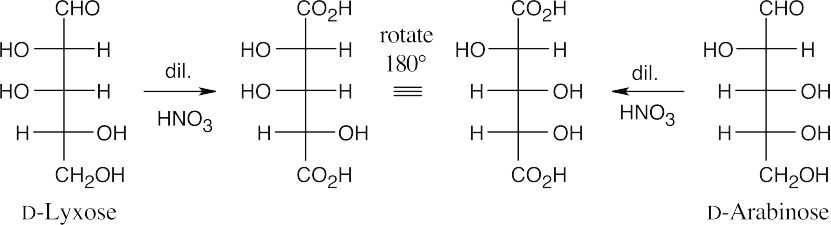 25.58
25.58
- (a)D-Galactose gives the same aldaric acid as L-galactose.
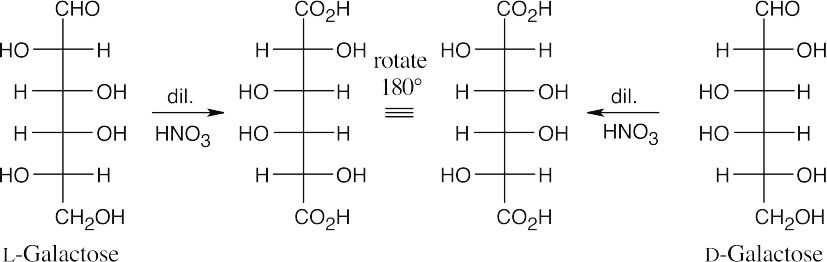
- The other aldohexose is a D-sugar. (c)
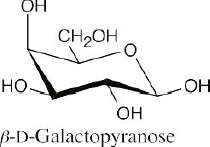
- Amygdalin has the same carbohydrate skeleton as gentiobiose (Problem 25.54). Draw the cyanohydrin of benzaldehyde, and form a bond between the hemiacetal oxygen and the carbonyl carbon of benzaldehyde, with elimination of water.
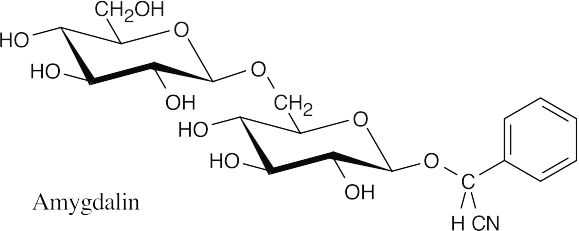
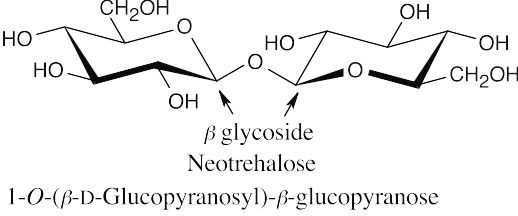
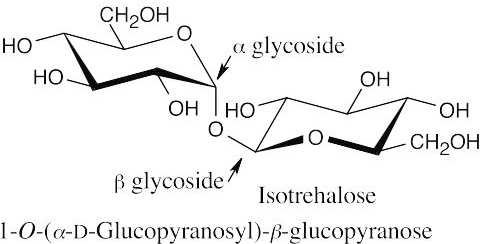
- Since trehalose is a nonreducing sugar, the two glucose units must be connected through an oxygen atom at the anomeric carbon of each glucose. There are three possible structures for trehalose: The two glucopyranose rings can be connected (α,α), (β, β), or (α,β).
- Since trehalose is not cleaved by β-glycosidases, it must have an α,α glycosidic linkage.
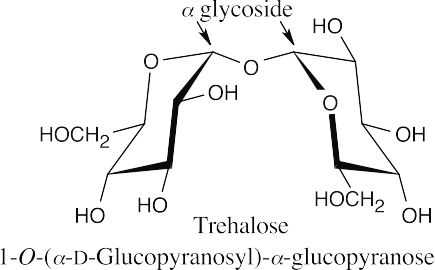
Acetone forms an acetal with the hydroxyl groups at C2 and C3 of D-mannopyranoside because the hydroxyl groups at these positions are cis to one another. The pyranoside ring is still a hemiacetal that is in equilibrium with free aldehyde, which is reducing toward Tollens’ reagent.
- Dilute base abstracts a proton α to the carbonyl carbon, forming an enolate. The enolate double bond can be protonated from either side, giving either mannose or glucose as the product.
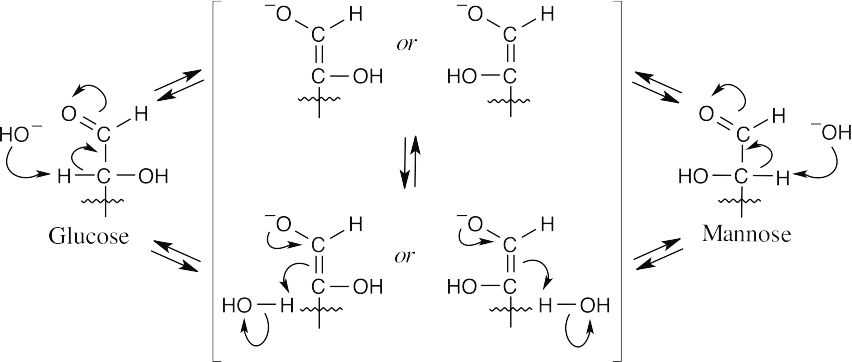
Isomerization at C2 occurs because the enediol can be reprotonated on either side of the double bond.
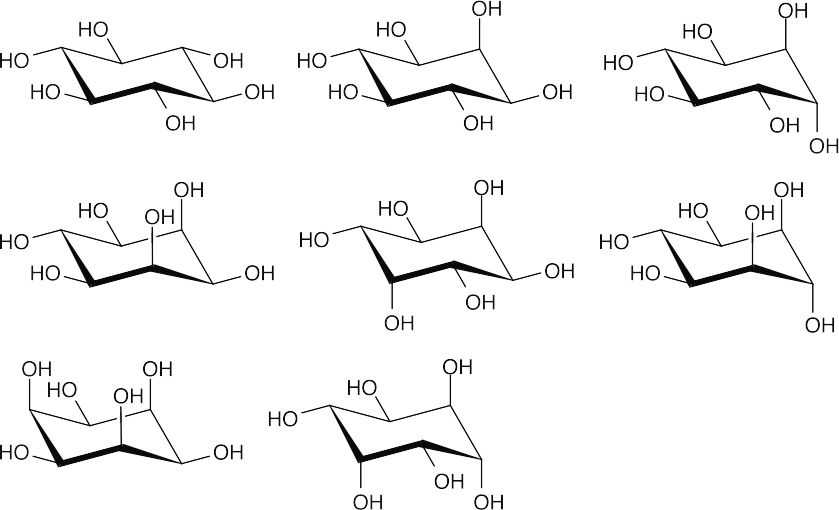
- There are eight diastereomeric cyclitols.
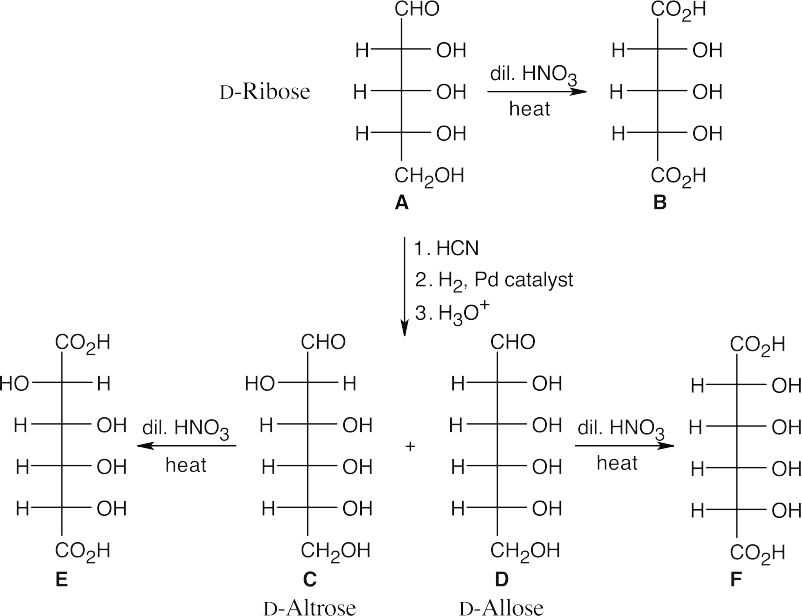
Because A is oxidized to an optically inactive aldaric acid, the possible structures are D- ribose and D-xylose. Chain extension of D-xylose, however, produces two hexoses that, when oxidized, yield optically active aldaric acids.
25.69 (a)
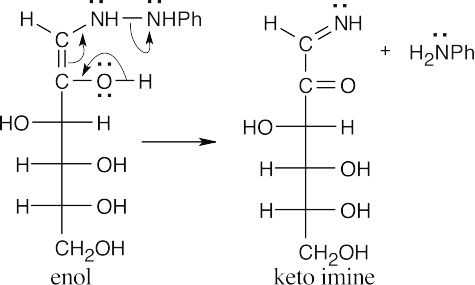 (b)(c)
(b)(c)
(d)
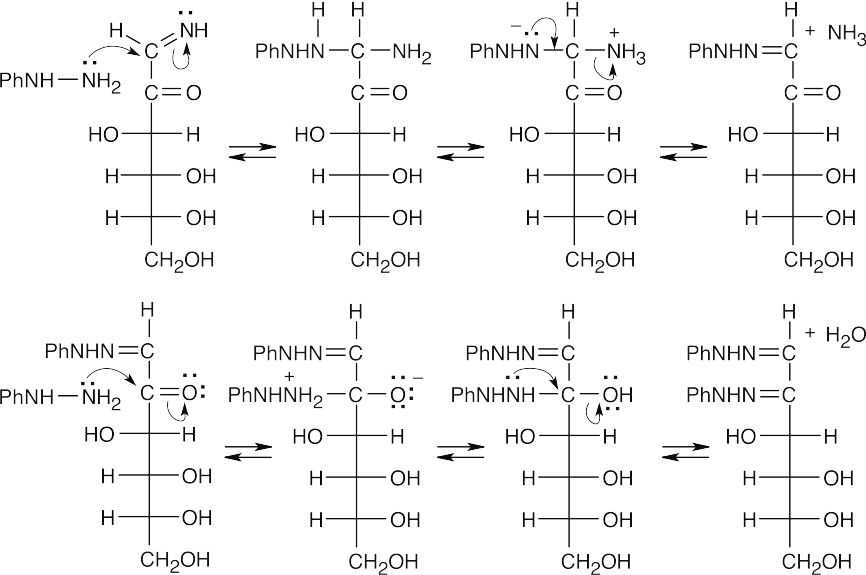
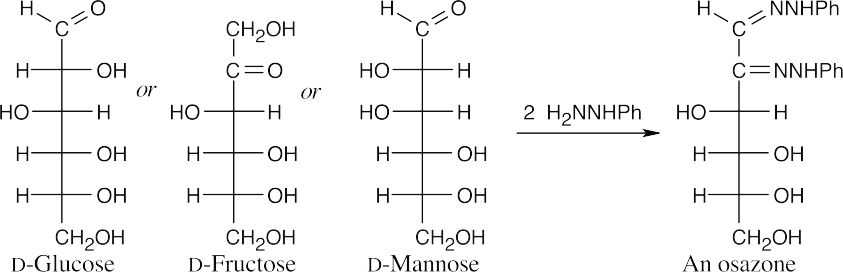
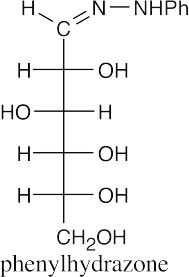
In these last steps, two nucleophilic addition reactions take place to yield imine products. The mechanism has been worked out in greater detail in Section 19.8, but the essential steps are additions of phenylhydrazine, first to the imine, then to the ketone. Proton transfers are followed by eliminations, first of ammonia, then of H2O.
25.70 (a)
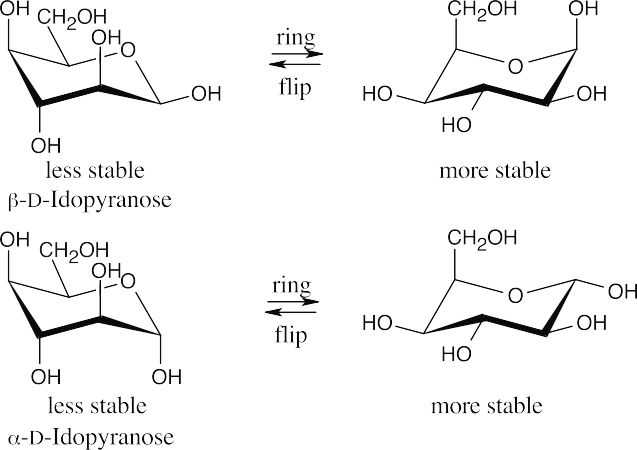
(b)α-D-Idopyranose is more stable than β-D-idopyranose because only one group is axial in its more stable chair conformation, whereas β-D-idopyranose has two axial groups in its more stable conformation.
(c)
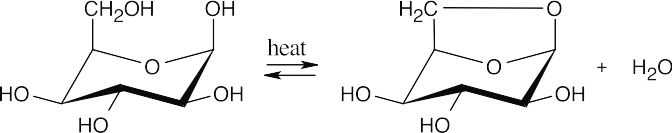
1,6-Anhydro-D-idopyranose is formed from the β anomer because the axial hydroxyl groups on carbons 1 and 6 are close enough for the five-membered ring to form.
(d)The hydroxyl groups at carbons 1 and 6 of D-glucopyranose are equatorial in the most stable conformation and are too far apart for a ring to form.
25.71
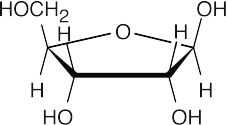
D-Ribofuranose is the sugar present in acetyl CoA.
This file is copyright 2023, Rice University. All Rights Reserved.