29 Chapter 29 – The Organic Chemistry of Metabolic Pathways
Solutions to Problems
29.1This reaction is a substitution at phosphorus, with ADP as the leaving group.
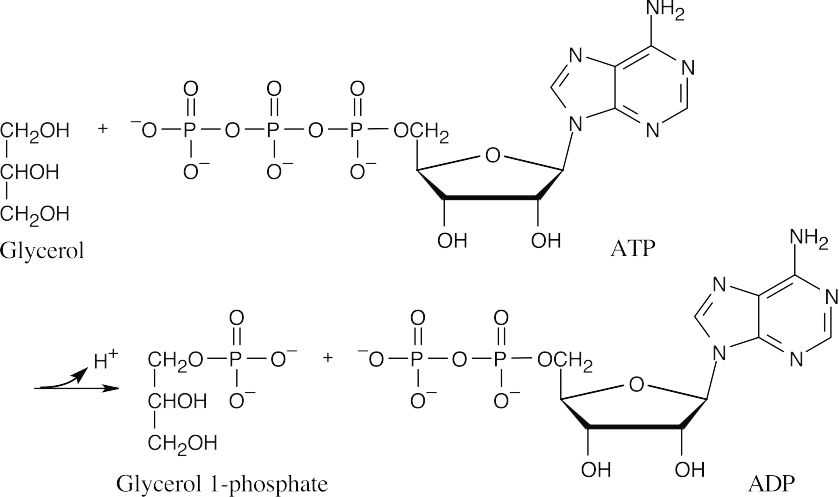
29.2
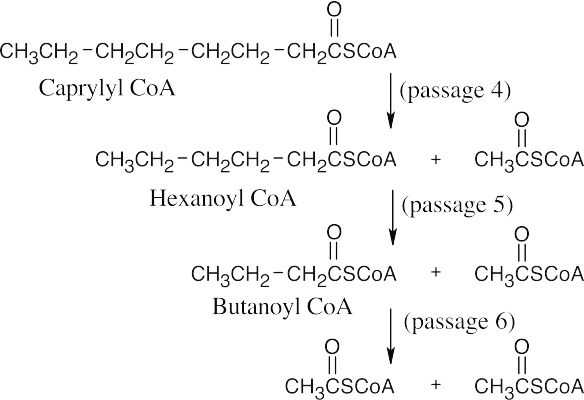
- A fatty acid with n carbons yields n/2 acetyl CoA molecules after (n/2 – 1) passages of the β-oxidation pathway.
(a)
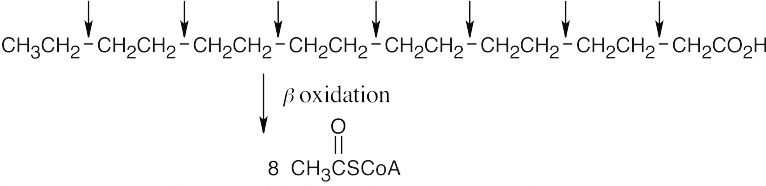
Seven passages of the β-oxidation pathway are needed.
(b)
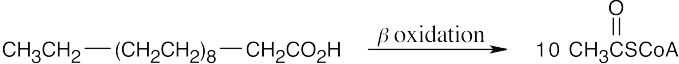
Nine passages of the β-oxidation pathway are needed.
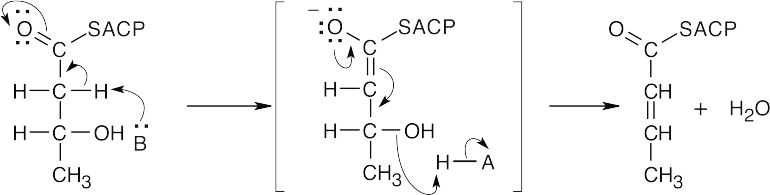
- β-Hydroxybutyryl ACP resembles the β-hydroxy ketones that were described in Chapter 23 and that dehydrate readily by an E1cB mechanism.
- A fatty acid synthesized from 13CH3CO2H has an alternating labeled and unlabeled carbon chain. The carboxylic acid carbon is unlabeled.
********
CH3CH2 CH2CH2 CH2CH2 CH2CH2 CH2CH2 CH2CH2 CH2CH2 CH2CO2H
- The face in front of the plane of the page is the Re face. Since addition occurs from behind the plane of the page, it occurs at the Si face.
- ATP is produced in step 7 (1,3-bisphosphoglycerate → 3-phosphoglycerate) and in step 10 (phosphoenolpyruvate → pyruvate). Refer to Figure 29.8.
- Step 1 is a nucleophilic acyl substitution at phosphorus (phosphate transfer) by the
–OH group at C6 of glucose, with ADP as the leaving group.
Step 2 is an isomerization, in which the pyranose ring of glucose 6-phosphate opens, tautomerism causes isomerization to fructose 6-phosphate, and a furanose ring is formed.
Step 3 is a substitution, similar to the one in step 1, involving the –OH group at C1 of fructose 6-phosphate (phosphate transfer).
Step 4 is a retro-aldol reaction that cleaves fructose 1,6-bisphosphate to glyceraldehydes 3-phosphate and dihydroxyacetone phosphate.
Step 5 is an isomerization of dihydroxyacetone phosphate to glyceraldehyde 3-phosphate that occurs by keto–enol tautomerization.
Step 6 begins with a nucleophilic addition reaction to the aldehyde group of glyceraldehyde 3-phosphate by a thiol group of an enzyme to form a hemithioacetal, which is oxidized by NAD+ to an acyl thioester. Nucleophilic acyl substitution by phosphate yields the product 1,3-bisphosphoglycerate.
Step 7 is a nucleophilic acyl substitution reaction at phosphorus, in which ADP reacts with 1,3-diphosphoglycerate, yielding ATP and 3-phosphoglycerate (phosphate transfer).
Step 8 is an isomerization of 3-phosphoglycerate to 2-phosphoglycerate.
Step 9 is an E1cB elimination of H2O to form phosphoenolpyruvate.
Step 10 is a substitution reaction at phosphorus that forms ATP and enolpyruvate, which tautomerizes to pyruvate (phosphate transfer).
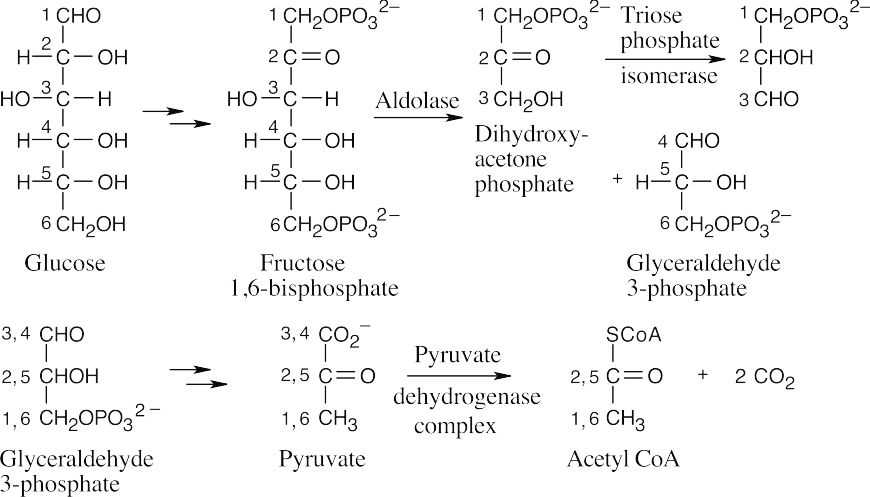
Carbons 1 and 6 of glucose end up as –CH3 groups of acetyl CoA, and carbons 3 and 4 of glucose end up as CO2.
- Citrate and isocitrate are tricarboxylic acids. Refer to Figure 29.14.
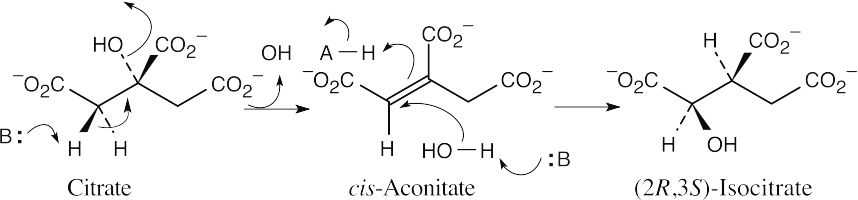
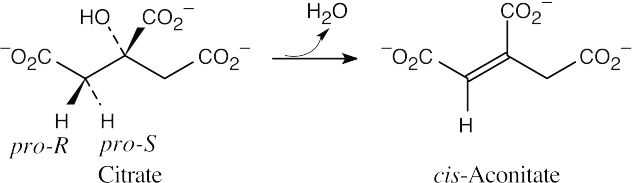
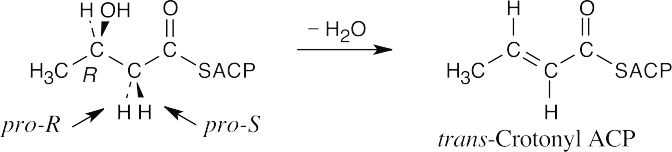
- The pro-R hydrogen is removed during dehydration, and the reaction occurs with anti
geometry.
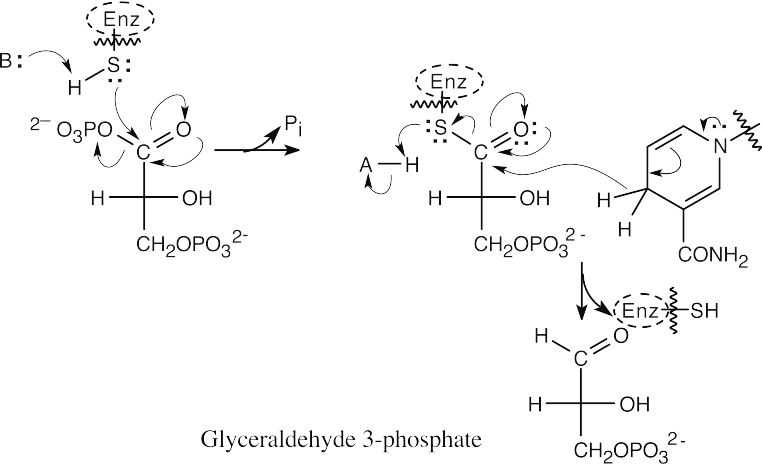
- First, 1,3-bisphosphoglycerate reacts with a cysteine residue of the enzyme in a nucleophilic acyl substitution reaction, with loss of phosphate. Then, reduction by NADH in a second nucleophilic acyl substitution reaction yields glyceraldehyde 3-phosphate.
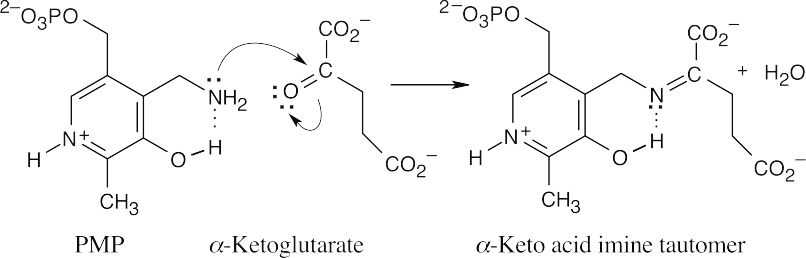
Nucleophilic acyl substitution, followed by loss of water, forms the imine tautomer.
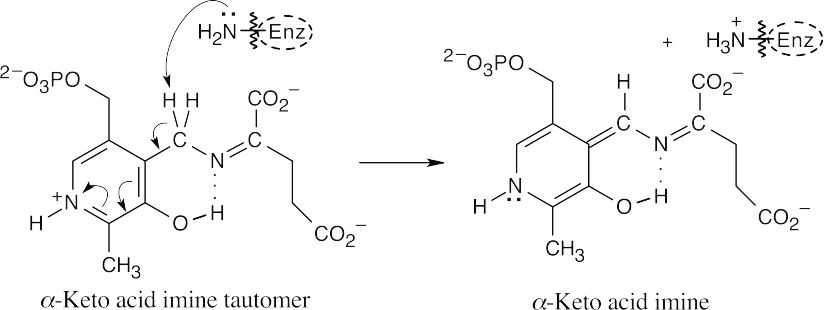
A lysine residue deprotonates the carbon next to the ring, leading to tautomerization.
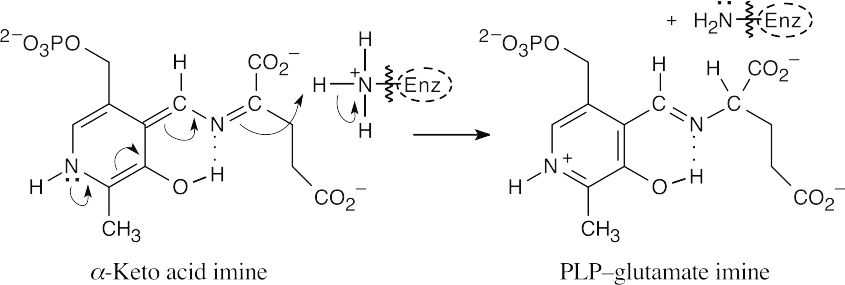
Enzymatic protonation of the keto acid imine yields PLP glutamate imine.
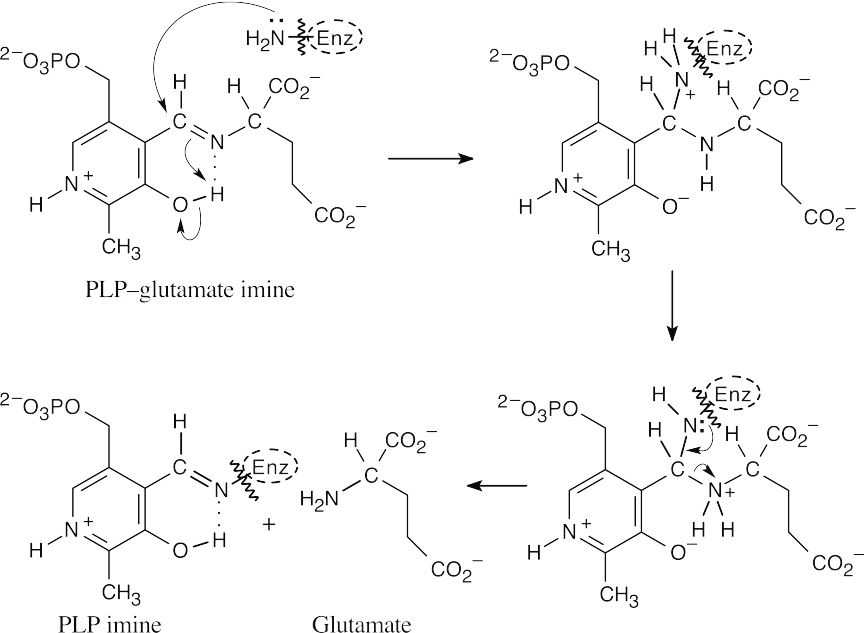
Addition of the enzyme, followed by loss of glutamate, regenerates PLP imine.
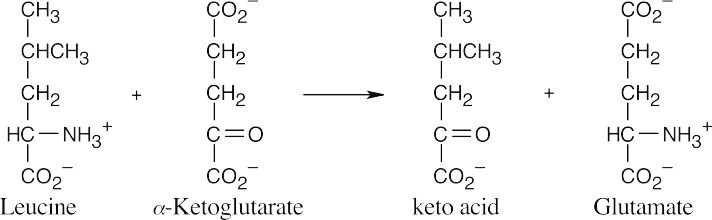
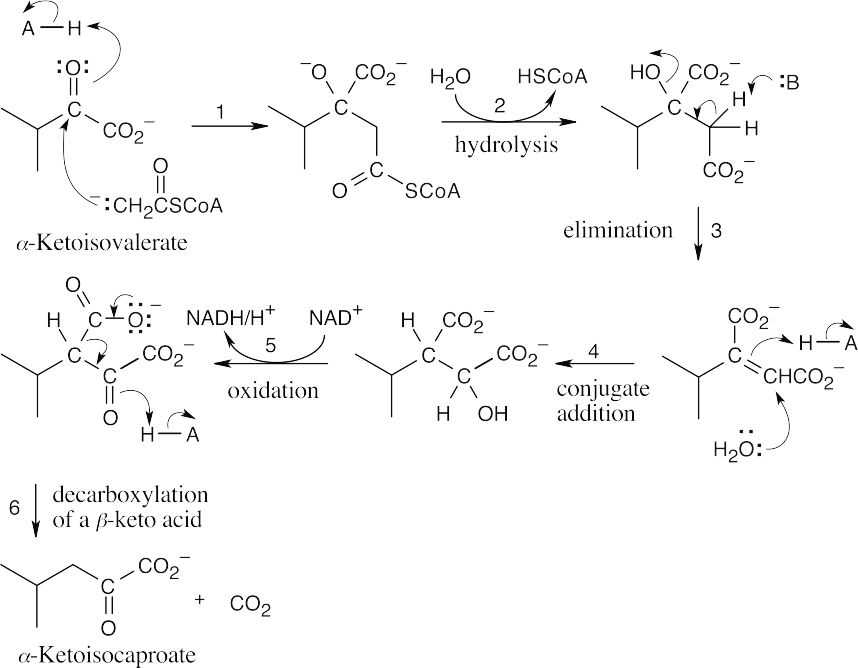
- Position leucine and α-ketoglutarate so that the groups to be exchanged are aligned. This arrangement makes it easy to predict the products of transamination reactions.
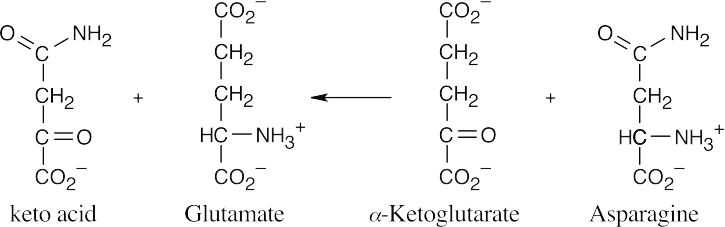
- As in the previous problem, redraw the α-keto acid and align it with glutamate. By exchanging the keto group and the amino group, you can identify the amino acid as asparagine.
Additional Problems
Visualizing Chemistry
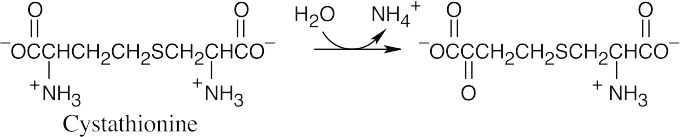
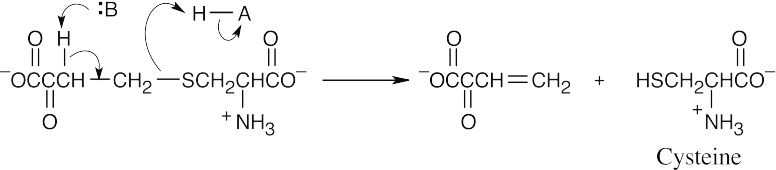
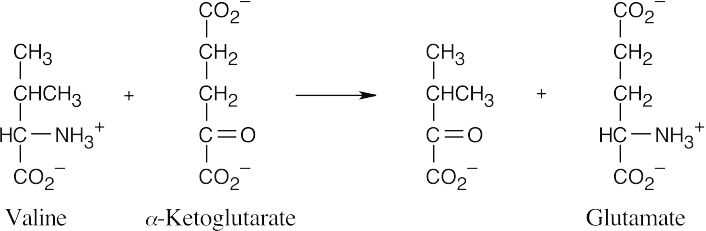 The amino acid precursors are valine (a) and methionine (b). (a)
The amino acid precursors are valine (a) and methionine (b). (a)
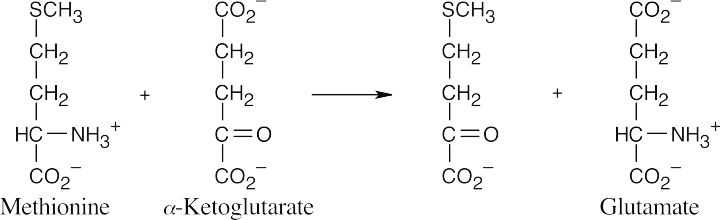 (b)
(b)
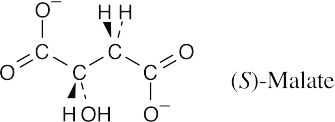
- The intermediate is (S)-malate. Refer to Figure 29.14.
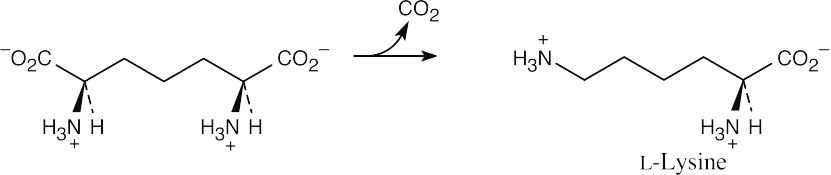
Decarboxylation of the intermediate yields lysine.
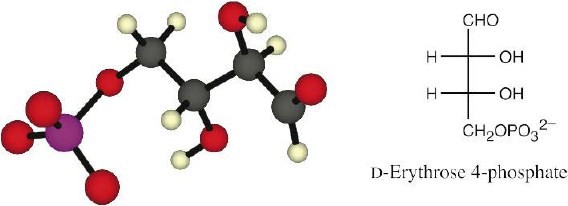 The intermediate is derived from D-erythrose.
The intermediate is derived from D-erythrose.
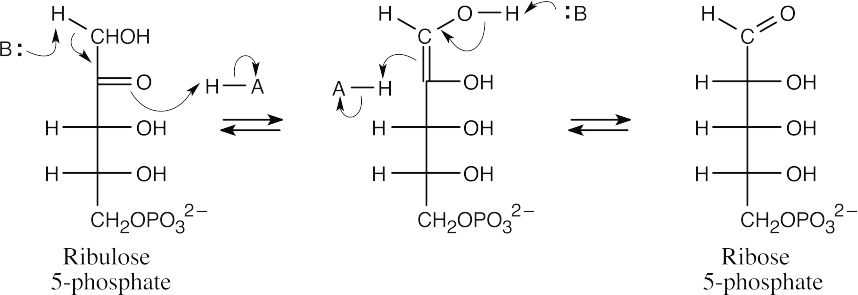 Mechanism Problems 29.21
Mechanism Problems 29.21
The isomerization of ribulose 5-phosphate to ribose 5-phosphate occurs by way of an intermediate enolate.
- This is a reverse aldol reaction, similar to step 4 of glycolysis.
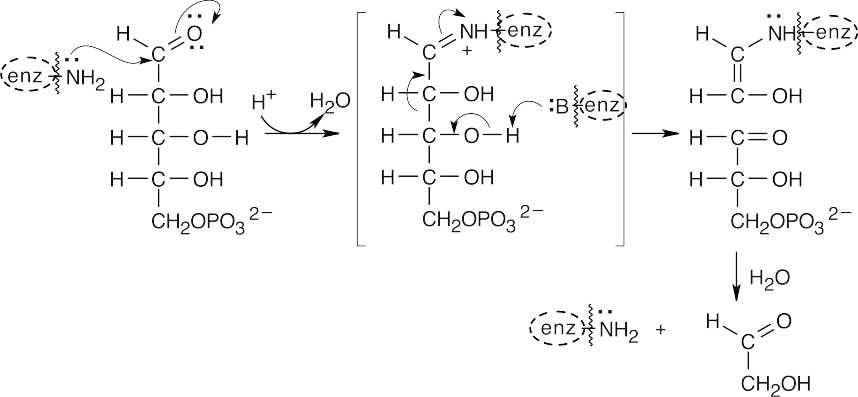
- (a)The first sequence of steps in this mechanism involves formation of the imine (Schiff base) of sedoheptulose 7-phosphate, followed by retro-aldol cleavage to form erythrose 4-phosphate and the enamine of dihydroxyacetone.
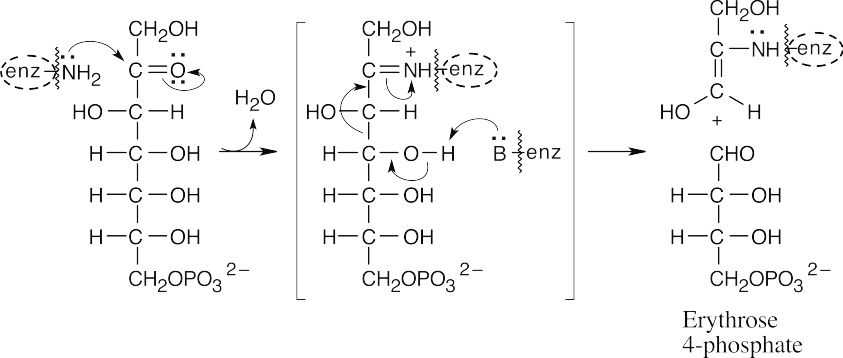
(b)The enamine of dihydroxyacetone adds to glyceraldehyde 3-phosphate to yield fructose 6-phosphate. This reaction is almost identical to the reaction pictured for Step 8 of gluconeogenesis in Section 29.8.
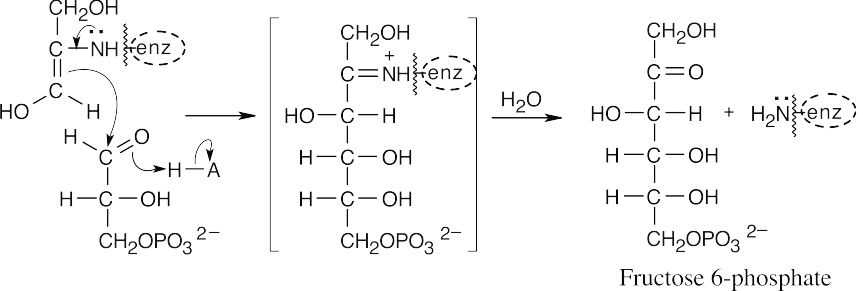
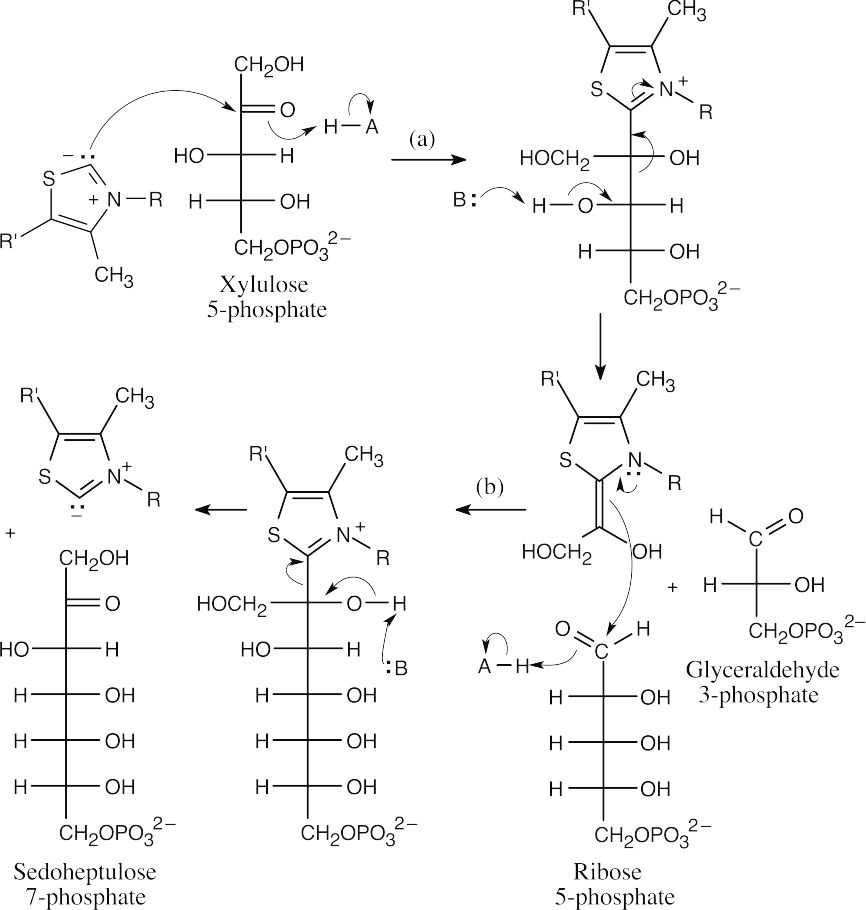
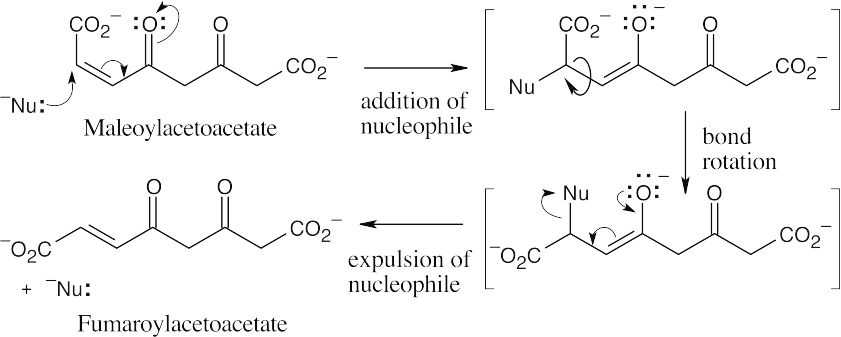
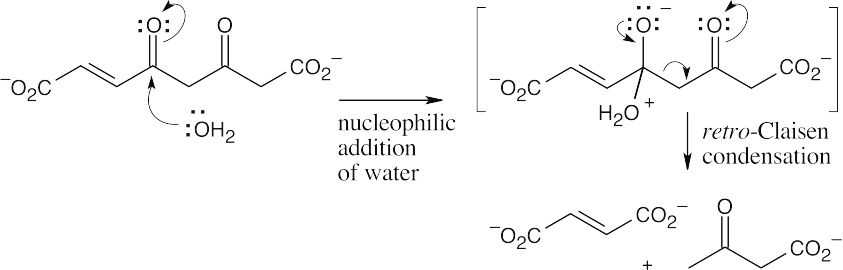
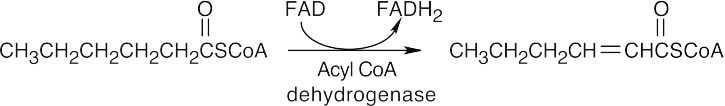
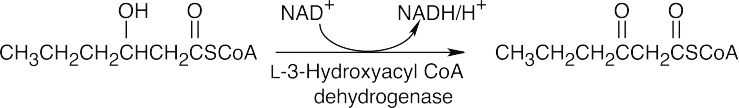
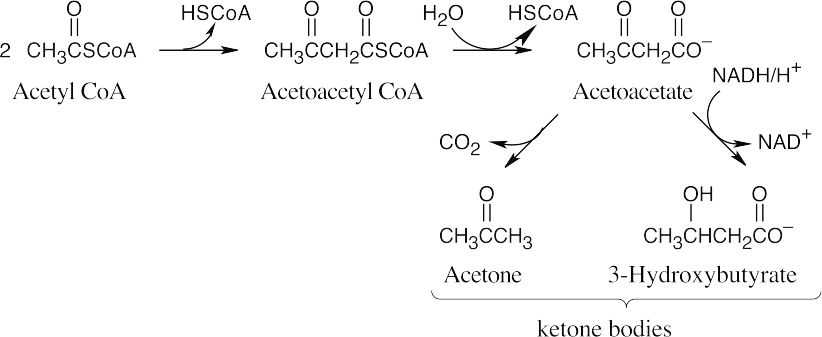
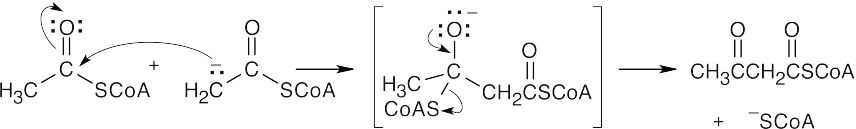
The first step in the conversion acetoacetateacetyl CoA is the formation of acetoacetyl CoA. This reaction also occurs as the first step in fatty acid catabolism. Although we haven’t studied the mechanism, it involves formation of a mixed anhydride.

The final step is a retro-Claisen reaction, whose mechanism is pictured in Section 29.3 as Step 4 of β-oxidation of fatty acids.
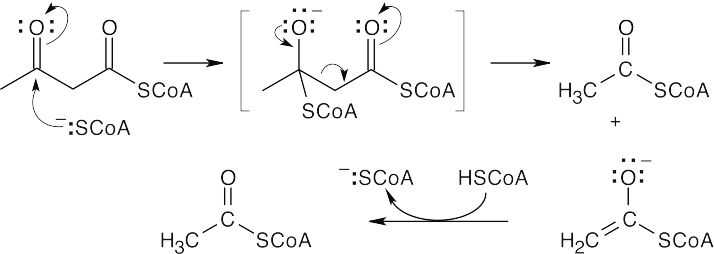
- Now is a good time to use retrosynthetic analysis, which was first encountered in Chapter
9. In this degradative pathway, what might be the precursor to acetyl CoA (the final product)? Pyruvate is a good guess, because we learned how to convert pyruvate to acetyl CoA in Section 29.6. How do we get from serine to pyruvate? A transamination reaction is a possibility. However, the immediate transamination precursor to pyruvate is the amino acid alanine, which differs from serine by one hydroxyl group. Thus, we probably have to design a pathway from serine to pyruvate that takes this difference into account.
Many routes are possible, but here’s the simplest:
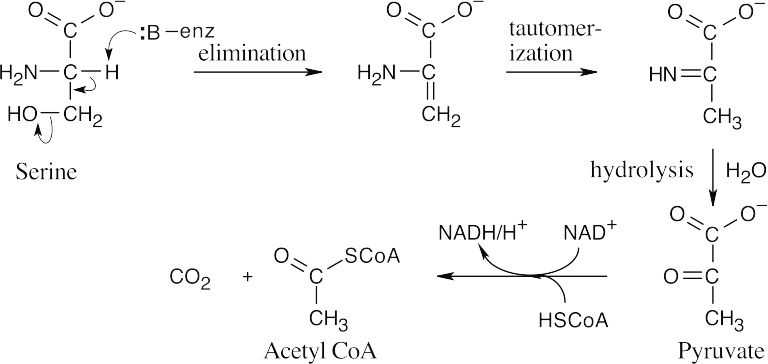
The coenzymes thiamin diphosphate and lipoamide are involved in the last step.
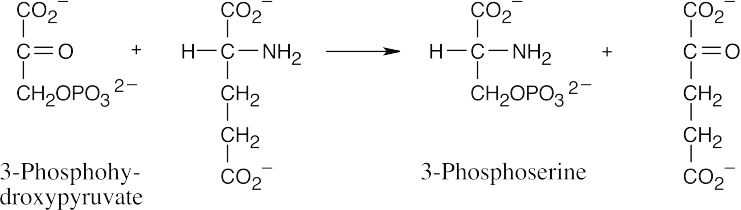
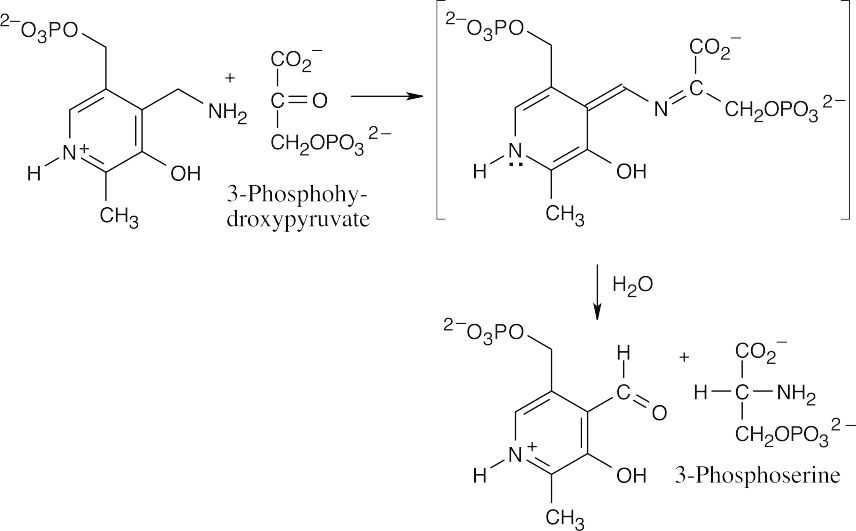
This reaction is a transamination that requires the coenzyme pyridoxal phosphate as a cofactor. The mechanism, which is described in Figure 29.17, involves two steps. The first step is the nucleophilic addition of glutamate nitrogen to the aldehyde group of pyridoxal phosphate to yield an imine intermediate, which is hydrolyzed to give α– ketoglutarate plus a nitrogen-containing pyridoxal phosphate byproduct.
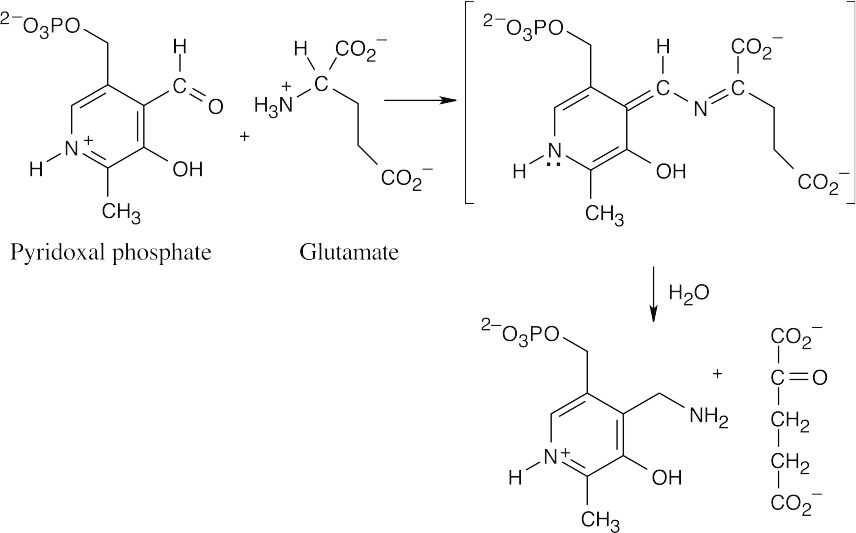
This byproduct reacts with 3-phosphohydroxypyruvate to give 3-phosphoserine plus regenerated pyridoxal phosphate.
 (a)
(a)
 (b)
(b)
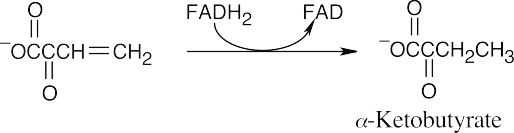 (c)
(c)
The product of double-bond reduction is α-ketobutyrate. FADH2 is the necessary coenzyme.
Enzymes and Coenzymes
- Digestion is the breakdown of bulk food in the stomach and small intestine. Hydrolysis of amide, ester and acetal bonds yields amino acids, fatty acids, and simple sugars, and these processes release energy.
- Metabolism refers to all reactions that take place inside cells. Digestion is a catabolic part of metabolism in which food is broken down into small organic molecules.
- Metabolic processes that break down large molecules are known as catabolism. Metabolic processes that assemble larger biomolecules from smaller ones are known as anabolism.
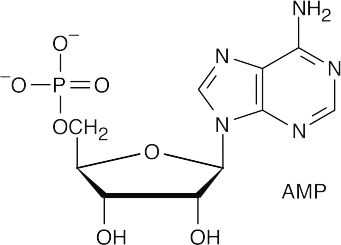
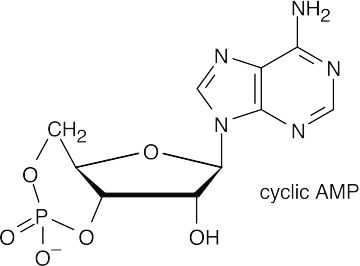
- ATP transfers a phosphate group to another molecule in anabolic reactions.
- NAD+ is a biochemical oxidizing agent that converts alcohols to aldehydes or ketones, yielding NADH and H+ as byproducts.
- FAD is an oxidizing agent that introduces a conjugated double bond into a biomolecule, yielding FADH2 as the reduced byproduct.
- (a)Pyridoxal phosphate is the cofactor associated with transamination.
- Biotin is the cofactor associated with carboxylation of a ketone.
- Thiamin diphosphate is the cofactor associated with decarboxylation of an α-keto acid.

NAD+ is needed to convert lactate to pyruvate because the reaction involves the oxidation of an alcohol.
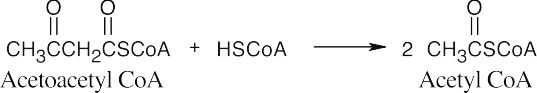 Metabolism 29.42
Metabolism 29.42
29.43
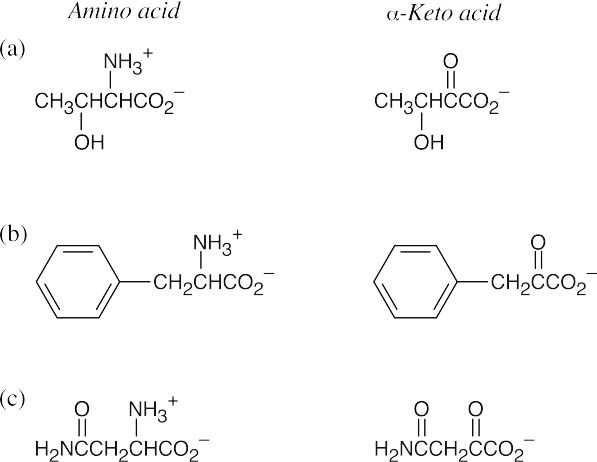
 (a)
(a)
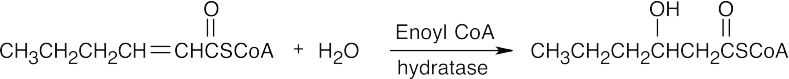 (b)
(b)
 (c)
(c)
- The exact reverse of an energetically favorable reaction is energetically unfavorable. Since glycolysis is energetically favorable (negative ΔG°’), its exact reverse has a positive ΔG°; and is energetically unfavorable. Instead, glucose is synthesized by gluconeogenesis, an alternate pathway that also has a negative ΔG°’.
- (a)One mole of glucose is catabolized to two moles of pyruvate, each of which yields one mole of acetyl CoA. Thus,
1.0 mol of glucose → 2.0 mol of acetyl CoA.
- A fatty acid with n carbons yields n/2 moles of acetyl CoA per mole of fatty acid. For palmitic acid (C15H31CO2H),
1.0 mol of palmitic acid×
8 mol of acetyl CoA 1 mol of palmitic acid
→ 8.0 mol of acetyl CoA.
- Maltose is a disaccharide that yields two moles of glucose on hydrolysis. Since each mole of glucose yields two moles of acetyl CoA,
1.0 mol of maltose → 2.0 mol of glucose → 4.0 mol of acetyl CoA.
|
29.46 |
|
|
|
(a) Glucose |
(b) Palmitic acid |
(c) Maltose |
|
Molecular weight180.2 amu |
256.4 amu |
342.3 amu |
|
Moles in 100.0 g0.5549 mol |
0.3900 mol |
0.2921 mol |
|
Moles of acetyl CoA2 × 0.5549 mol produced= 1.110 mol |
8 × 0.3900 mol = 3.120 mol |
4 × 0.2921 mol = 1.168 mol |
|
Grams acetyl CoA898.6 g |
2526 g |
945.6 g |
produced
Palmitic acid is the most efficient precursor of acetyl CoA on a weight basis.
29.47
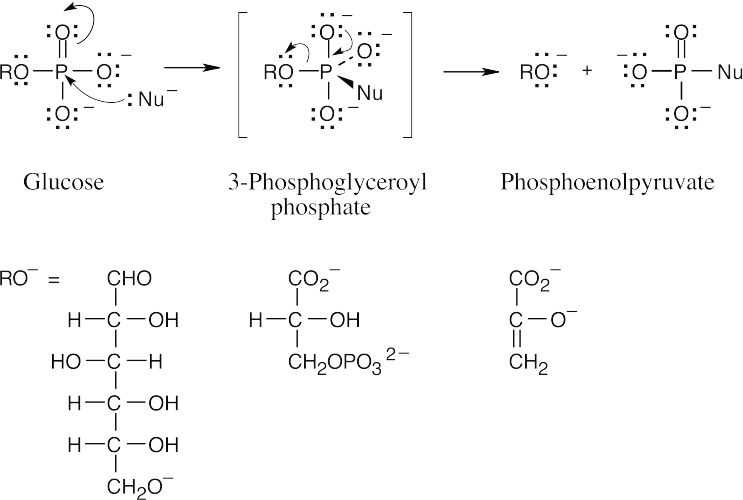
- As we saw in Section 29.1, formation of glucose 6-phosphate from glucose and ATP is energetically favorable (negative ΔG°’). The reverse reaction, transfer of a phosphate group to ADP from glucose 6-phosphate, is energetically unfavorable and doesn’t occur spontaneously. In chemical terms, the leaving groups in the reactions of 3-phosphoglyceroyl phosphate (carboxylate) and phosphoenolpyruvate (enolate) are more stable anions than the leaving group in the reaction of glucose (alkoxide), so these reactions are more favorable.
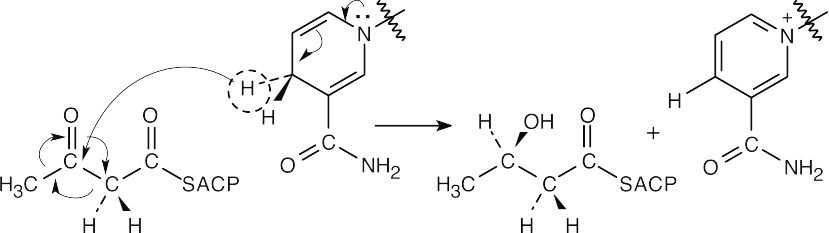
- The steps in the conversion of α-ketoglutarate to succinyl CoA are similar to steps in the conversion of pyruvate to acetyl CoA shown in Figure 29.13, and the same coenzymes are involved: lipoamide, thiamin diphosphate, acetyl CoA and NAD+.
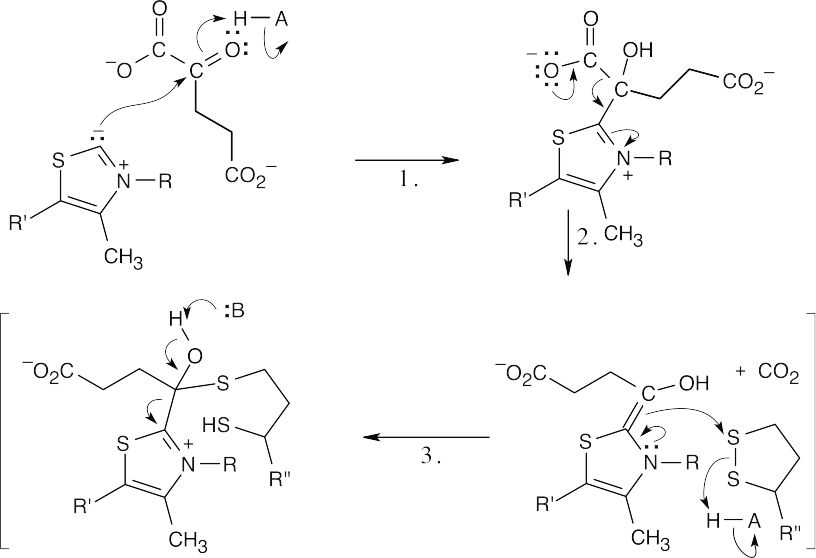
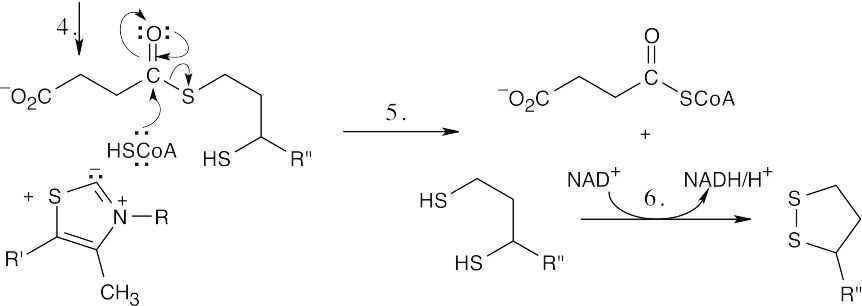
Step 1:Nucleophilic addition of thiamin diphosphate ylid.
Step 2:Decarboxylation.
Step 3:Addition of double bond to lipoamide, with ring opening.
Step 4:Elimination of thiamin diphosphate.
Step 5:Nucleophilic addition of acetyl CoA to succinyl lipoamide and elimination of dihydrolipoamide to give succinyl CoA.
Step 6:Reoxidation of dihydrolipoamide to lipoamide.
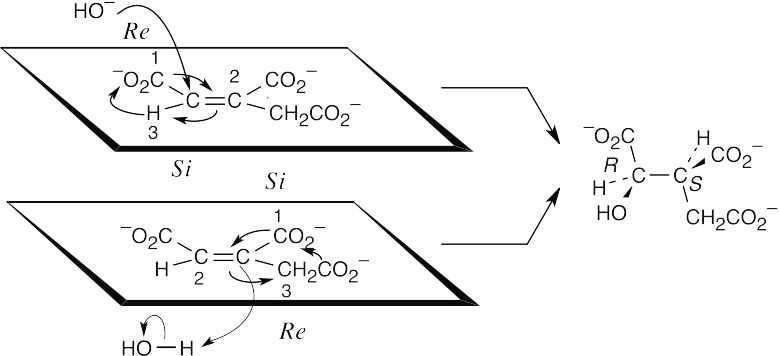
- Addition of –OH: For carbon 2, the top face is the Re face, and –OH adds from this face to give an R configuration at carbon 2.
Addition of H+: For carbon 3, the top face is Si, and H+ adds from the bottom, or Re, face to give an S configuration at carbon 3. The reaction occurs with anti geometry.
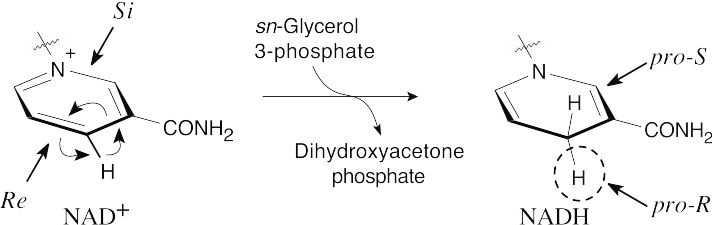 General Problems 29.51
General Problems 29.51
The face above the plane of the ring is the Si face. Since the problem states that addition of –H takes place from the Re face, the circled hydrogen comes from sn-glycerol. The added hydrogen has pro-R stereochemistry.
29.52
29.53
- Addition occurs from the Si face to form the R enantiomer.
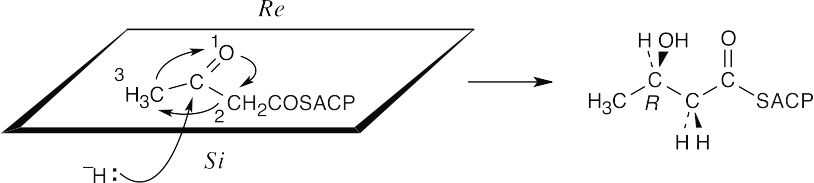
- If dehydration removes the pro-R hydrogen and the resulting double bond is trans, as indicated, anti elimination must have taken place.
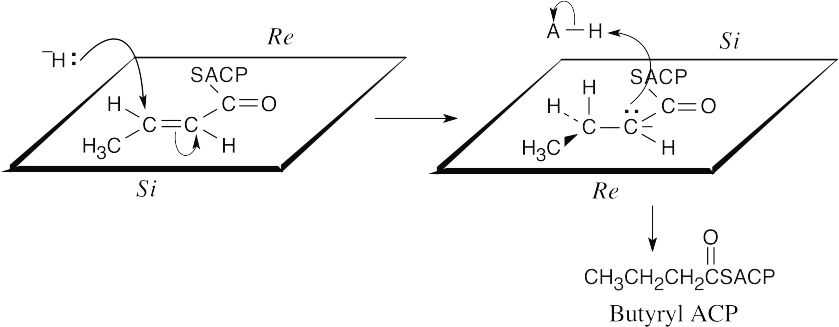
The reduction is a syn addition.
This file is copyright 2023, Rice University. All Rights Reserved.

