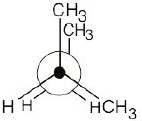
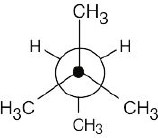
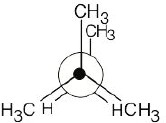
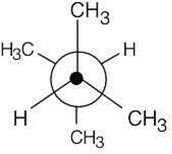
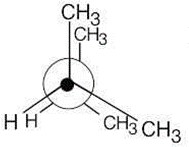
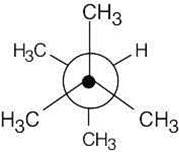
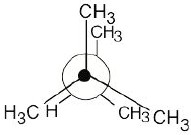
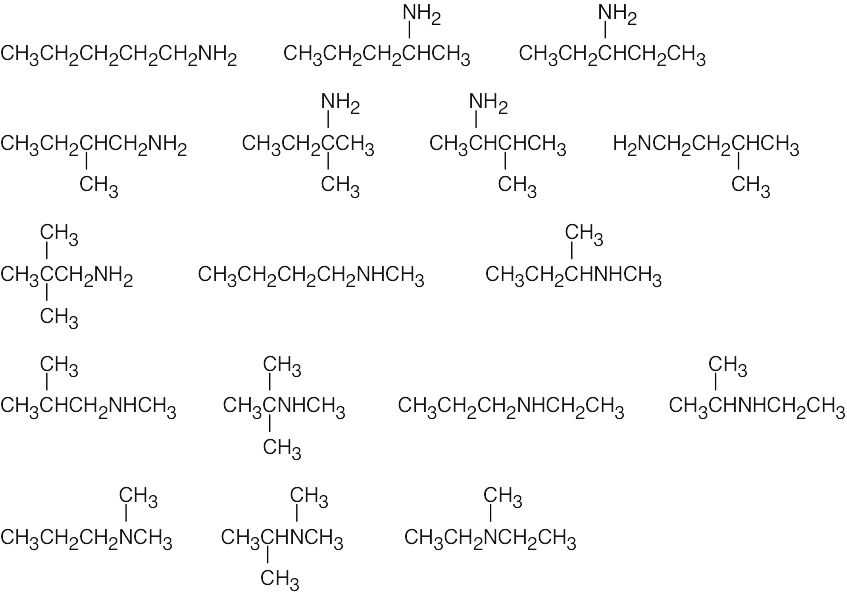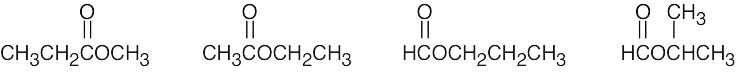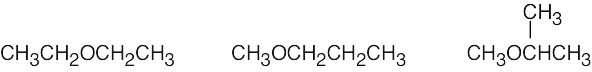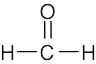3 Chapter 3 – Organic Compounds: Alkanes and Their Stereochemistry Solutions to Problems
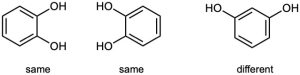
| 3.1 | Notice that certain functional groups have different designations if other functional groups are also present in a molecule. For example, a molecule containing a carbon–carbon double bond and no other functional group is an alkene; if other groups are present, the group is referred to as a carbon–carbon double bond. Similarly, a compound containing a benzene ring, and only carbon- and hydrogen-containing substituents, is an arene; if other groups are present, the ring is labeled an aromatic ring. | |
| (a) |  |
|
| (b) | 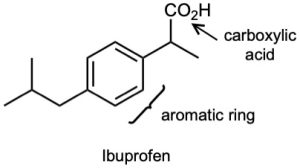 |
|
| (c) | 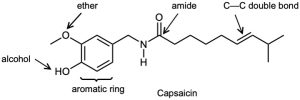 |
|
| 3.2 | (a) | 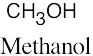 |
(d) | 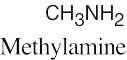 |
| (b) | 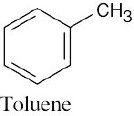 |
(e) | 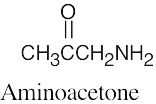 |
|
| (c) | 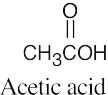 |
(f) | 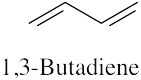 |
| 3.3 | 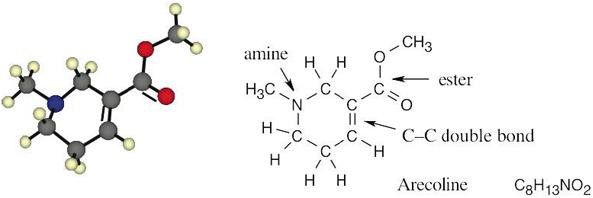 |
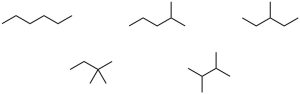
| 3.4 | We know that carbon forms four bonds and hydrogen forms one bond. Thus, draw all possible six-carbon skeletons and add hydrogens so that all carbons have four bonds. To draw all possible skeletons in this problem: (1) Draw the six-carbon straight-chain skeleton; (2) Draw a five-carbon chain, identify the different types of carbon atoms on the chain, and add a –CH3 group to each of the different types of carbons, generating two skeletons; (3) Repeat the process with the four-carbon chain to give rise to the last two skeletons. Add hydrogens to the remaining carbons to complete the structures.
|
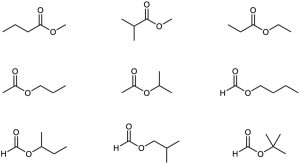
| 3.5 | (a) | Nine isomeric esters of formula C5H10O2 can be drawn. The procedure is described in Problem 3.4.
|
| (b) | Two isomers can be drawn.
|
|
| (c) | Three isomers can be drawn.
|
| 3.6 | (a) | Two alcohols have the formula C3H8O.
|
| (b) | Four bromoalkanes have the formula C4H9Br.
|
|
| (c) | Four thioesters have the formula C4H8OS.
|
| 3.7 | 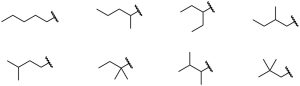 |
| 3.8 | (a) | 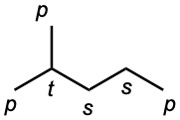 |
| (b) | 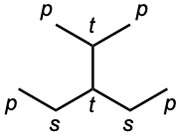 |
|
| (c) | 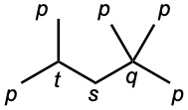 |
| 3.9 | The carbons and the attached hydrogens have the same classification. | |
| (a) |  |
|
| (b) |  |
|
| (c) |  |
|
| 3.10 | (a) | 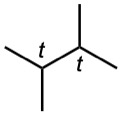 |
| (b) | 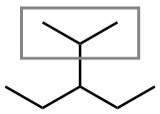 |
|
| (c) | 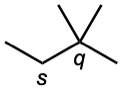 |
| 3.11 | (a) | 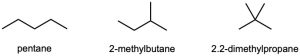 |
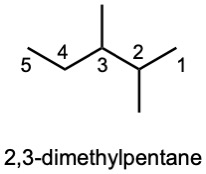
| (b) | Step 1: Find the longest continuous carbon chain and use it as the parent name. In (b), the chain is a pentane.
Step 2: Identify the substituents. In (b), both substituents are methyl groups. Step 3: Number the substituents. In (b), the methyl groups are in the 2- and 3- positions. Step 4: Name the compound. Remember that the prefix di– must be used when two are the same. The IUPAC name is 2,3-dimethylpentane.
|
|
| (c) | 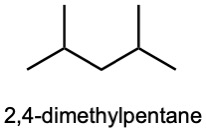 |
|
| (d) | 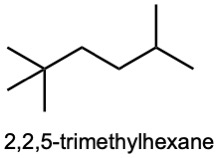 |
| 3.12 | When you are asked to draw the structure corresponding to a given name, draw the parent carbon chain, attach the specified groups to the proper carbons, and fill in the remaining hydrogens. | |
| (a) | 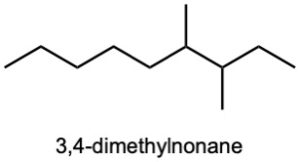 |
|
| (b) | 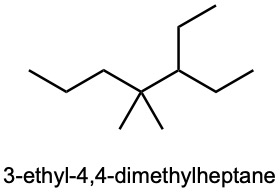 |
|
| (c) | 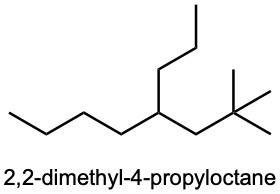 |
|
| (d) | 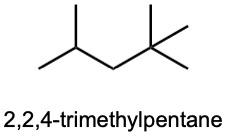 |
|
| 3.13 | 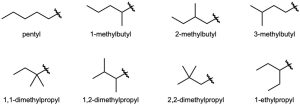 |
| 3.14 | 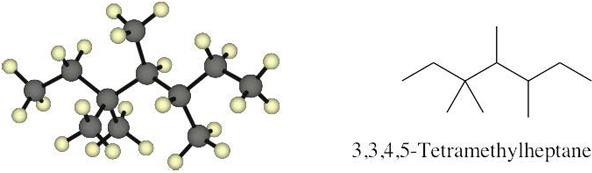 |
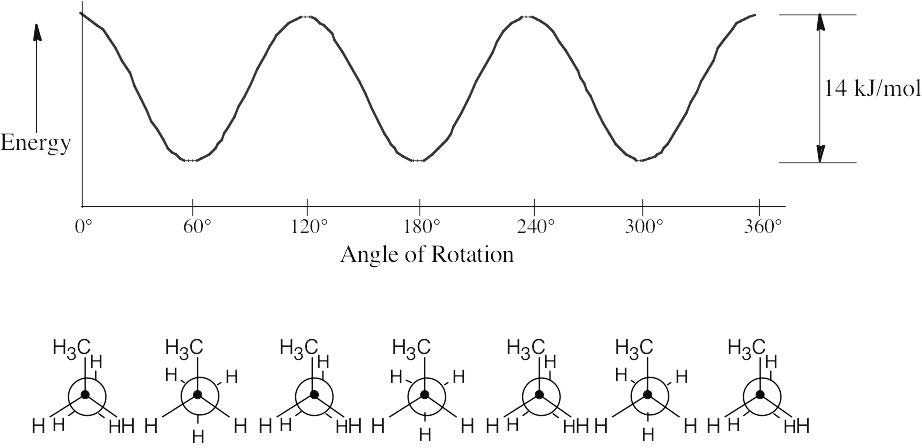
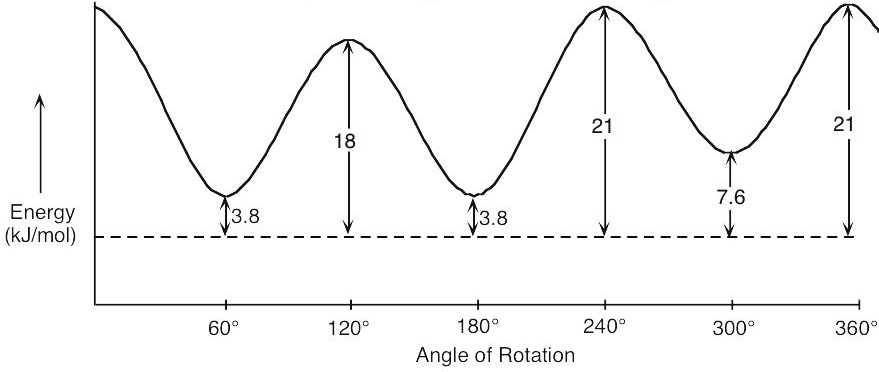
| 3.15 | The graph shows the energy of a conformation as a function of angle of rotation.
|
| 3.16 | (a) |  |
| (b) | 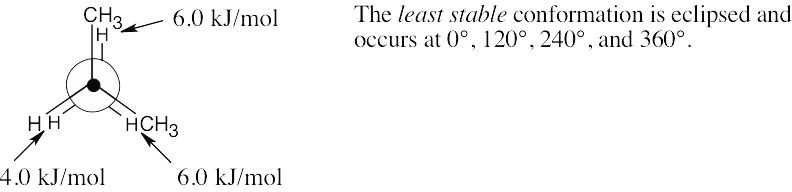 |
|
| (c), (d) | 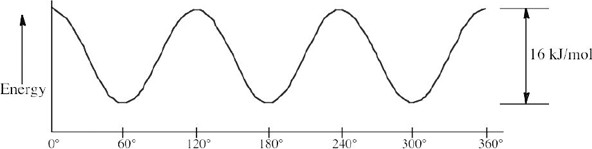 |
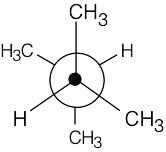
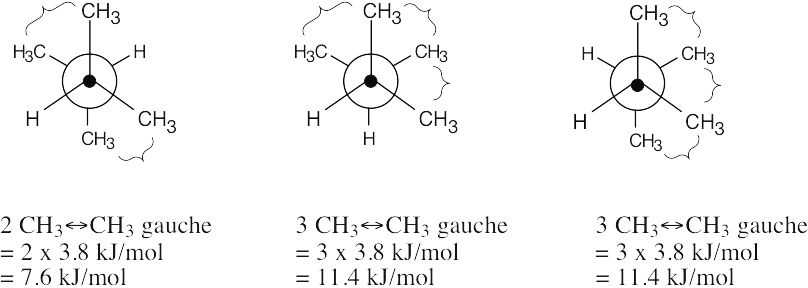
| 3.17 | This conformation of 2,3-dimethylbutane is the most stable because it is staggered and has the fewest CH3↔CH3 gauche interactions.
|
| 3.18 | The conformation is a staggered conformation in which the hydrogens on carbons 2 and 3 are 60° apart. Draw the Newman projection.
|
Additional Problems
Visualizing Chemistry 3.19
| 3.19 | (a) | 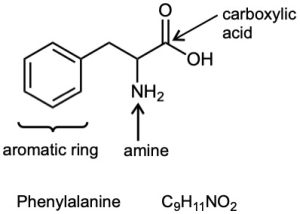 |
| (b) | 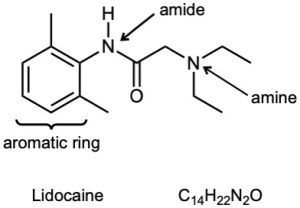 |
| 3.20 | (a) | 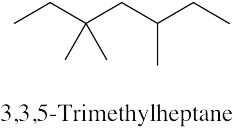 |
| (b) | 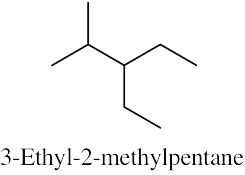 |
|
| (c) | 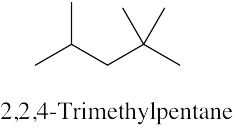 |
|
| (d) | 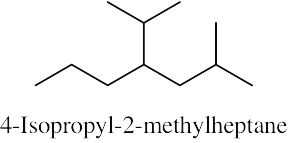 |
| 3.21 | In this conformation, all groups are staggered and the two methyl groups are 180° apart.
|
Functional Groups
| 3.22 | (a) | 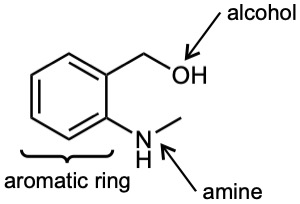 |
(d) | 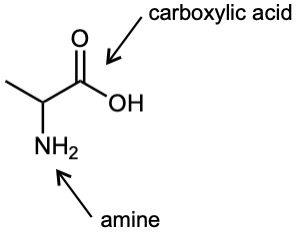 |
| (b) | 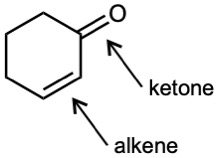 |
(e) | 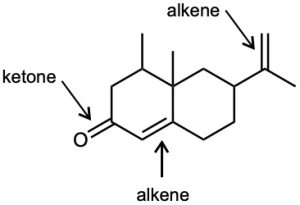 |
|
| (c) | 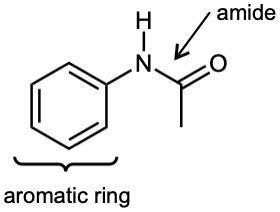 |
(f) | 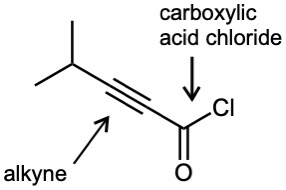 |
| 3.23 | Different answers to this problem and to Problem 3.24 are acceptable. | |||
| (a) | (d) | 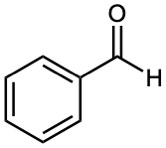 |
||
| (b) | 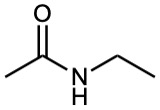 |
(e) | 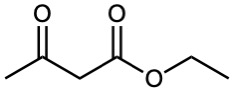 |
|
| (c) | (f) | |||
| 3.24 | For (a) and (h), only one structure is possible. | |||
| (a) | (e) | |||
| (b) | (f) | 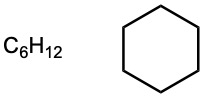 |
||
| (c) | 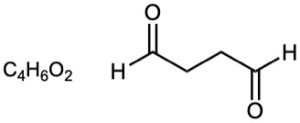 |
(g) | ||
| (d) | (h) | |||
| 3.25 | (a) | 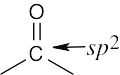 |
| (b) | 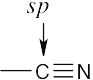 |
|
| (c) | 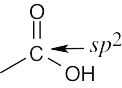 |
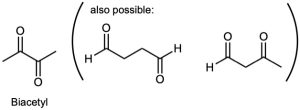
| 3.26 | (a) | Although it is stated that biacetyl contains no rings or carbon–carbon double bonds, it is obvious from the formula for biacetyl that some sort of multiple bond must be present. The structure for biacetyl contains two carbon–oxygen double bonds.
|
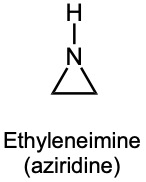
| (b) | Ethyleneimine contains a three-membered ring.
|
|
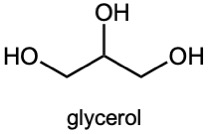
| (c) | Glycerol contains no multiple bonds or rings.
|
Isomers
| 3.27 | (a) | Eighteen isomers have the formula C8H18. Three are pictured.
|
| (b) | Structures with the formula C4H8O2 may represent esters, carboxylic acids or many other complicated molecules. Three possibilities:
|
| 3.28 | 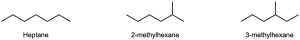 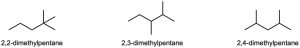 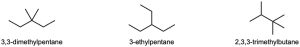 |
| 3.29 | (a) | 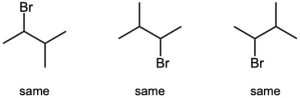 |
| (b) | Give the number “1” to the carbon bonded to –OH, and count to find the longest chain containing the –OH group.
|
|
| (c) | 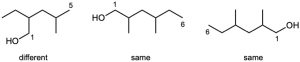 |
| 3.30 | The isomers may be either alcohols or ethers.
|
| 3.31 | First, draw all straight-chain isomers. Then proceed to the simplest branched structure. | |
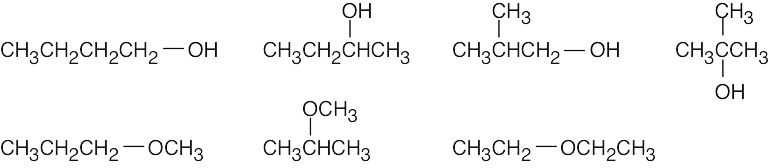
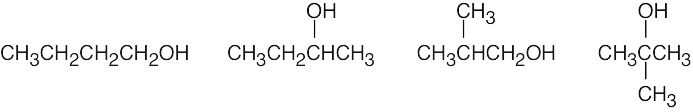
| (a) | There are four alcohol isomers with the formula C4H10O.
|
|
| (b) | There are 17 isomers of C5H13N. Nitrogen can be bonded to one, two or three alkyl groups.
|
|
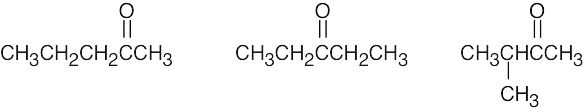
| (c) | There are 3 ketone isomers with the formula C5H10O.
|
|
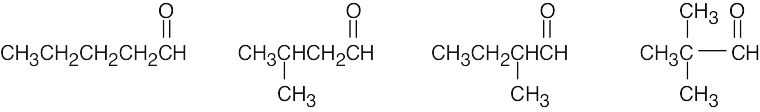
| (d) | There are 4 isomeric aldehydes with the formula C5H10O. Remember that the aldehyde functional group can occur only at the end of a chain.
|
|
| (e) | There are 4 esters with the formula C4H8O2.
|
|
| (f) | There are 3 ethers with the formula C4H10O.
|
|
| 3.32 | (a) | 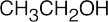 |
(d) | 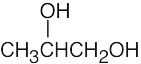 |
| (b) | 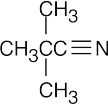 |
(e) | 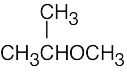 |
|
| (c) | 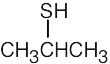 |
(f) | 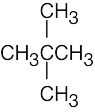 |
Naming Compounds
| 3.33 | 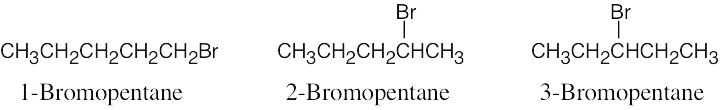 |
| 3.34 | 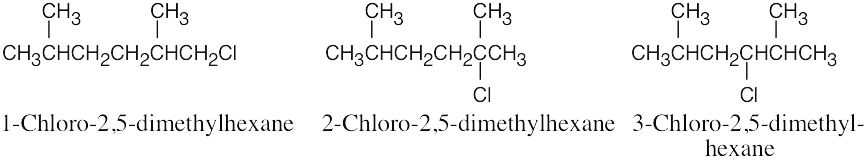 |
| 3.35 | (a) | 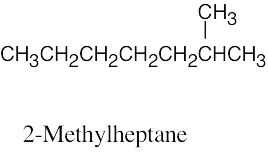 |
(d) | 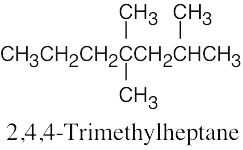 |
| (b) | 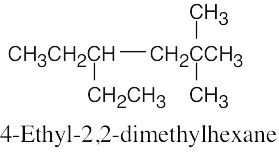 |
(e) | 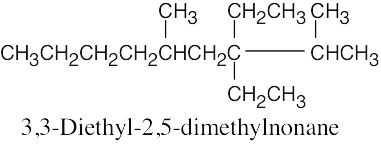 |
|
| (c) | 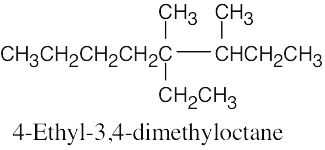 |
(f) | 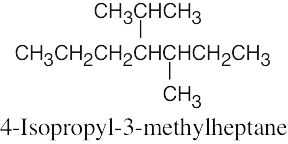 |
| 3.36 | (a) | 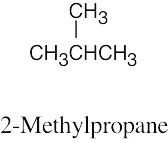 |
| (b) | 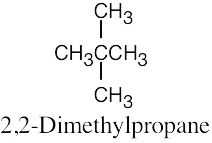 |
|
| (c) | 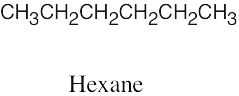 |
| 3.37 | (a) | 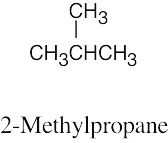 |
| (b) |  |
| 3.38 | (a) | 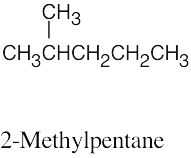 |
(d) | 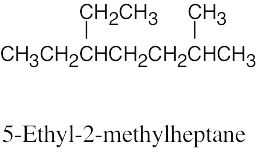 |
| (b) | 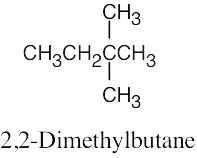 |
(e) | 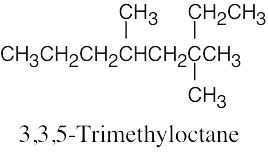 |
|
| (c) | 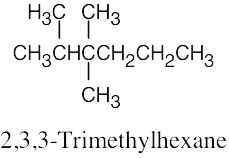 |
(f) | 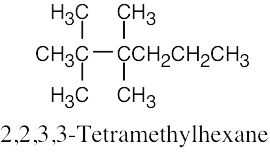 |
| 3.39 | 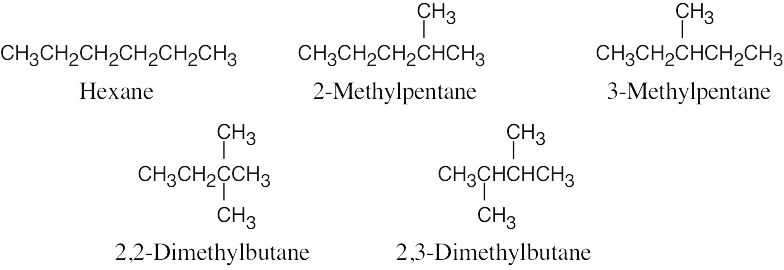 |
| 3.40 | Structure and Correct Name | Error | |
| (a) | 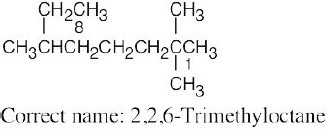 |
The longest chain is an octane and has only methyl branches. | |
| (b) | 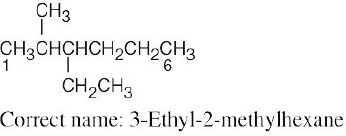 |
The longest chain is a hexane. Numbering should start from the opposite end of the carbon chain, nearer the first branch. | |
| (c) | 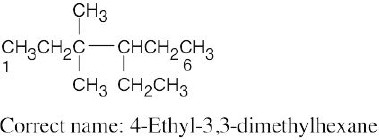 |
Numbering should start from the opposite end of the carbon chain. See step 2(b) in Section 3.4. | |
| (d) | 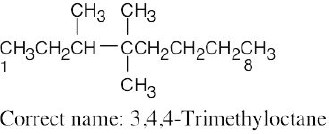 |
Numbering should start from the opposite end of the carbon chain. | |
| (e) | 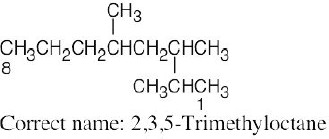 |
The longest chain is an octane. |
| 3.41 | (a) | 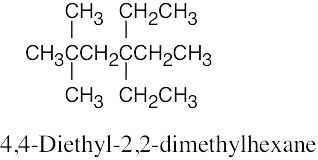 |
| (b) | 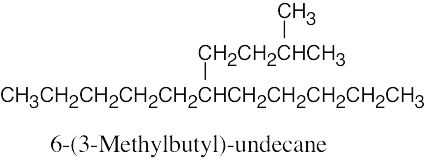
Remember that you must choose an alkane whose principal chain is long enough so that the substituent does not become part of the principal chain. |
Conformations
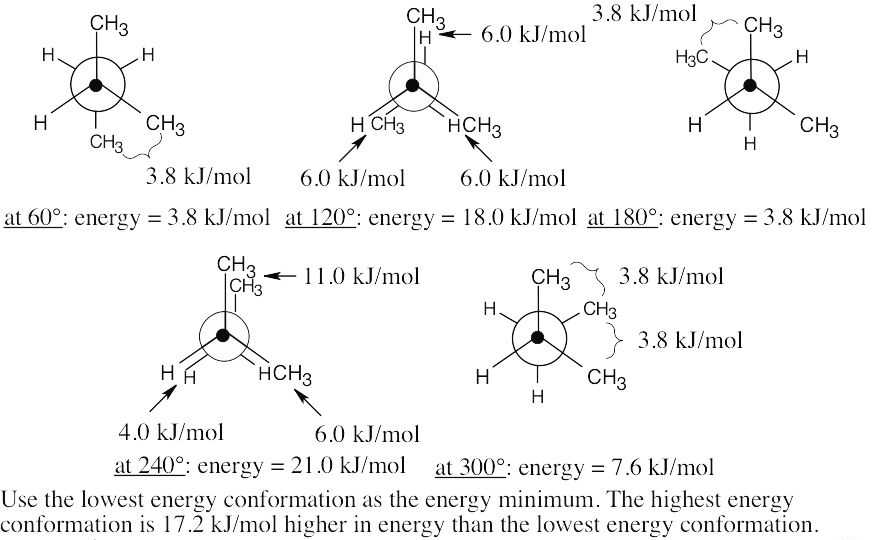
| 3.42 | (a), (b) | 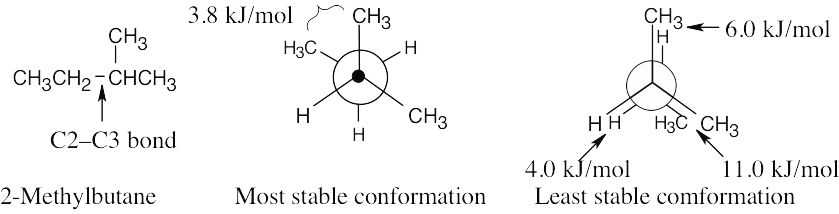
The energy difference between the two conformations is (11.0 + 6.0 + 4.0) kJ/mol – 3.8 kJ/mol = 17.2 kJ/mol. |
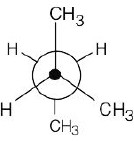
| (c) | Consider the least stable conformation to be at zero degrees. Keeping the front of the projection unchanged, rotate the back by 60° to obtain each conformation.
|
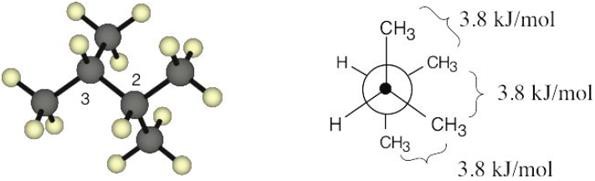
| 3.43 | Each CH3↔CH3 gauche interaction has a value of 3.8 kJ/mol.
|
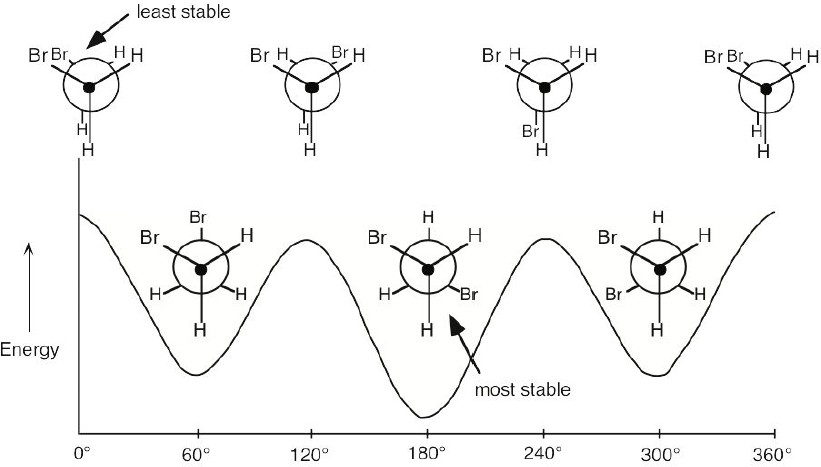
| 3.44 | Since we are not told the values of the interactions for 1,2-dibromoethane, the diagram can only be qualitative.
The anti conformation is at 180°. The gauche conformations are at 60°, 300°. |
| 3.45 | The eclipsed conformation at 0° rotation has the largest dipole moment but is a high energy that is present in low abundance. The anti conformation has no net dipole because the polarities of the individual bonds cancel. The gauche conformation, however, has a dipole moment. Because the observed dipole moment is 1.0 D at room temperature, a mixture of gauche and anti conformations must be present. |
| 3.46 | The best way to draw pentane is to make a model and to copy it onto the page. A model shows the relationship among atoms, and its drawing shows these relationships in two dimensions. From your model, you should be able to see that all atoms are staggered in the drawing.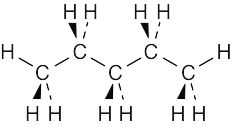 |
| 3.47 | 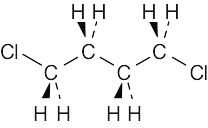 |
General Problems
| 3.48 | (a) | 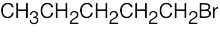 |
(d) | 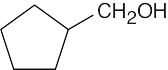 |
| (b) | 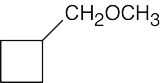 |
(e) | 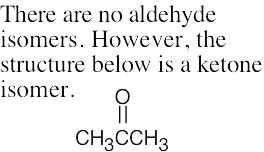 |
|
| (c) | 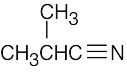 |
(f) | 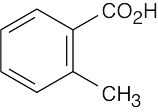 |
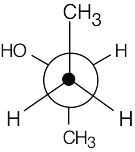
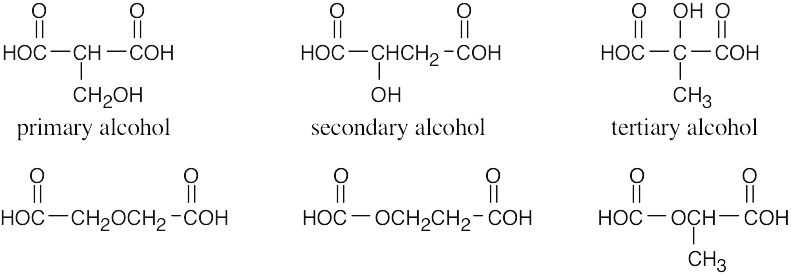
| 3.49 | (a) | Because malic acid has two –CO2H groups, the formula for the rest of the molecule is C2H4O. Possible structures for malic acid are:
|
| (b) | Because only one of these compounds (the second one) is also a secondary alcohol, it must be malic acid. |
| 3.50 | To solve this type of problem, read the problem carefully, word for word. Then try to interpret parts of the problem. For example: | |
| 1) | Formaldehyde is an aldehyde,
|
|
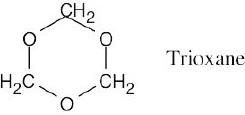
| 2) | It trimerizes – that is, 3 formaldehydes come together to form a compound C3H6O3. Because no atoms are eliminated, all of the original atoms are still present. | |
| 3) | There are no carbonyl groups. This means that trioxane cannot contain any –C=O functional groups. If you look back to Table 3.1, you can see that the only oxygen- containing functional groups that can be present are either ethers or alcohols. | |
| 4) | A monobromo derivative is a compound in which one of the –H’s has been replaced by a –Br. Because only one monobromo derivative is possible, we know that there can only be one type of hydrogen in trioxane. The only possibility for trioxane is:
|
|
| 3.51 | The highest energy conformation of bromoethane has a strain energy of 15 kJ/mol. Because this includes two H–H eclipsing interactions of 4.0 kJ/mol each, the value of an H–Br eclipsing interaction is 15 kJ/mol – 2(4.0 kJ/mol) = 7.0 kJ/mol.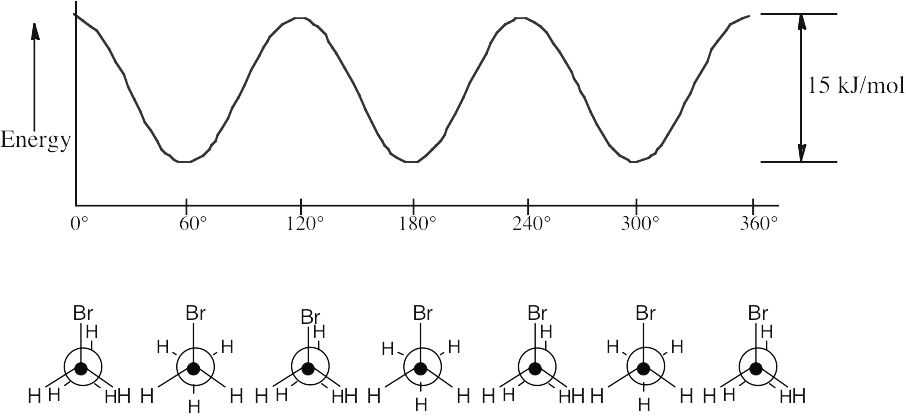 |
| 3.52 |
|
Most stable |
Strain energy |
Least stable |
Strain energy |
|
(a) |
|
* 3.8 kJ/mol |
|
21 kJ/mol |
|
|
(b) |
|
7.6 kJ/mol |
|
23 kJ/mol |
|
|
(c) |
|
7.6 kJ/mol |
|
26 kJ/mol |
|
|
(d) |
|
15.2 kJ/mol |
|
** 28 kJ/mol |
|
|
|
*most stable overall (least strain)
**least stable overall (most strain) |
||||
| 3.53 | The carboxylic acid and alcohol groups in Pravachol are an ester (lactone) group in Zocor. The alcohol at the bottom left of Pravachol is a methyl group in Zocor.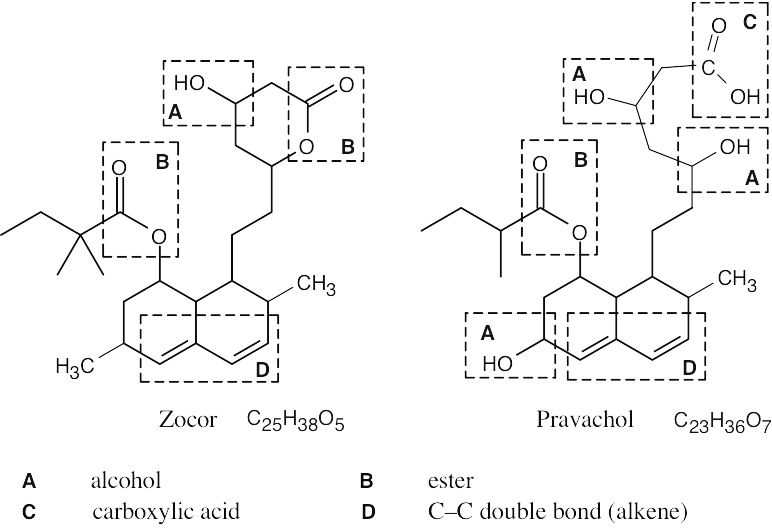 |
| 3.54 | A puckered ring allows all the bonds in the ring to have a nearly tetrahedral bond angle. (If the ring were flat, C–C–C bond angles would be 120°.) Also, a puckered conformation relieves strain due to eclipsed hydrogens.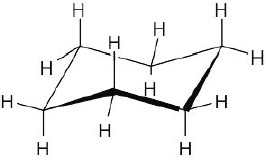 |
| 3.55 | In one of the 1,2-dimethylcyclohexanes the two methyl groups are on the same side of the ring, and in the other isomer the methyl groups are on opposite sides. |
This file is copyright 2023, Rice University. All Rights Reserved.