31 Chapter 31 – Synthetic Polymers Solutions to Problems
31.1
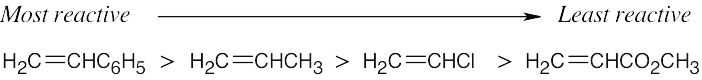
The alkenes most reactive to cationic polymerization contain electron-donating functional groups that can stabilize the carbocation intermediate. The reactivity order of substituents in cationic polymerization is similar to the reactivity order of substituted benzenes in electrophilic aromatic substitution reactions.
31.2
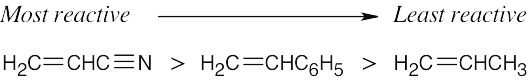
Anionic polymerization occurs most readily with alkenes having electron-withdrawing substituents.
31.3
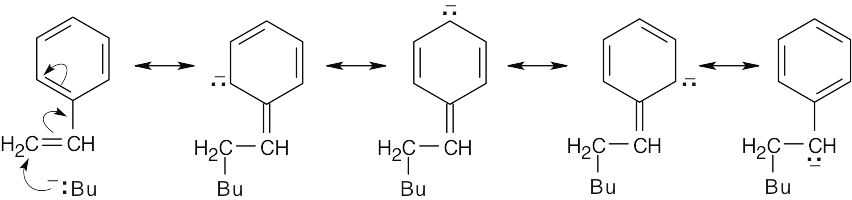
The intermediate anion can be stabilized by resonance involving the phenyl ring.
31.4

Vinylidene chloride doesn’t polymerize in isotactic, syndiotactic or atactic forms because
no asymmetric centers are formed during polymerization.
31.5None of the polypropylenes rotate plane-polarized light. If an optically inactive reagent and an achiral compound react, the product must be optically inactive. For every chirality center generated, an enantiomeric chirality center is also generated, and the resulting polymer mixture is optically inactive.
31.6
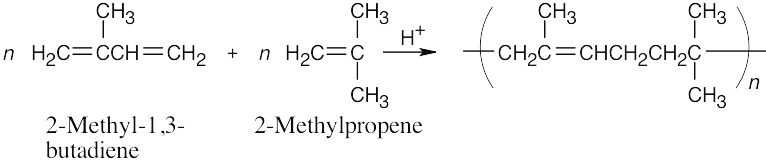
31.7
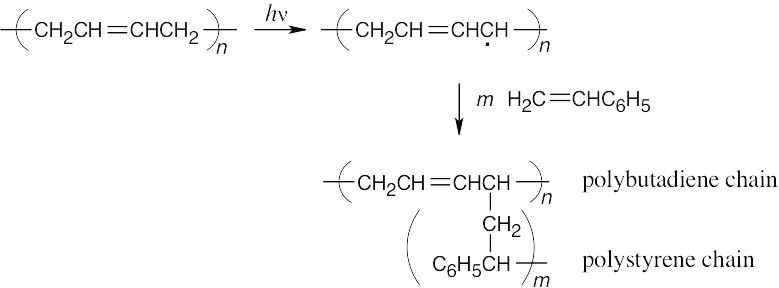
Irradiation homolytically cleaves an allylic C–H bond because it has the lowest bond energy. The resulting radical adds to styrene to produce a polystyrene graft.
31.8
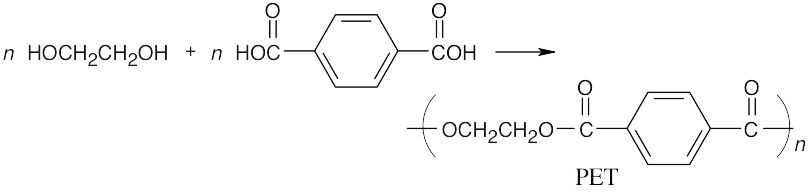
31.9
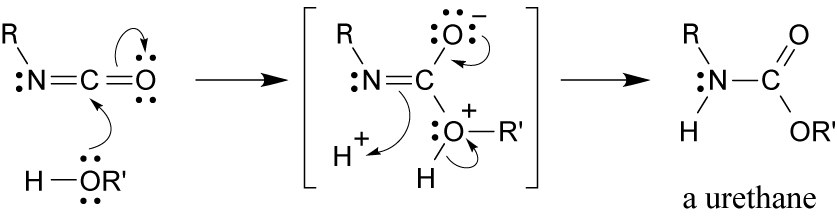
31.10
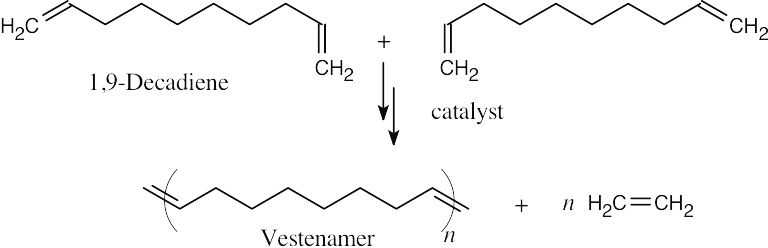
Vestenamer can be formed by an ADMET synthesis using 1,9-decadiene.
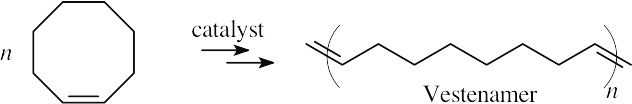
Vestenamer is usually synthesized from cyclooctene by a ROMP polymerization.
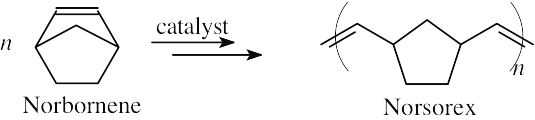
Norbornene undergoes ROMP polymerization to yield Norsorex.
31.11
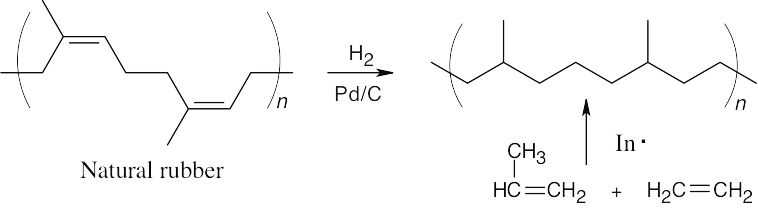
The product of hydrogenation of natural rubber is atactic. This product also results from the radical copolymerization of propene with ethylene.
31.12
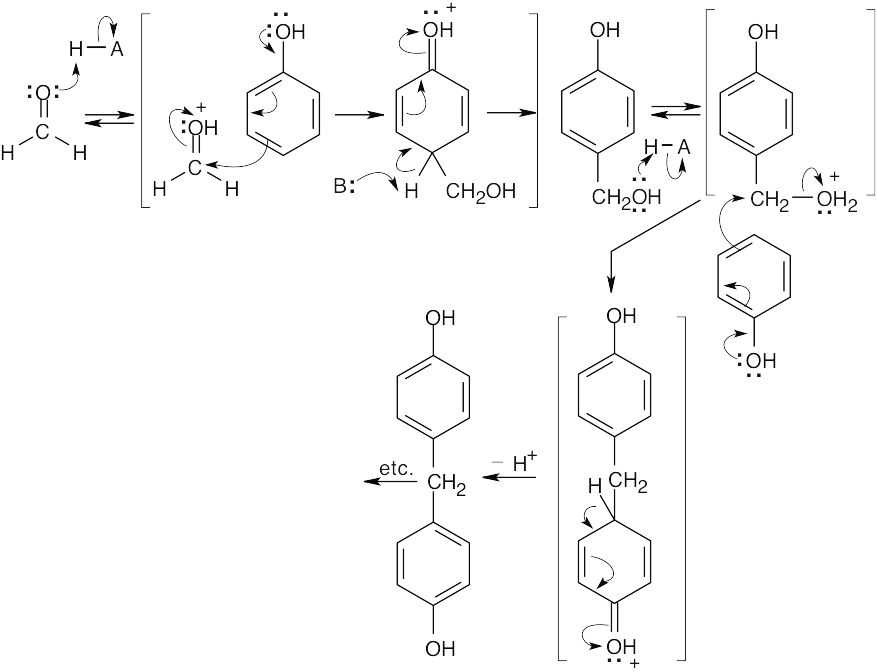
This product, formed by electrophilic aromatic substitution, can react many times with additional formaldehyde and phenol to yield Bakelite. Reaction occurs at both ortho and para positions of phenol.
Additional Problems
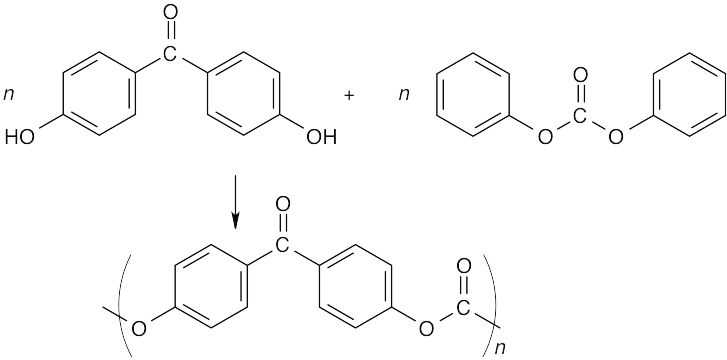 Visualizing Chemistry 31.13
Visualizing Chemistry 31.13
The polymer is a polycarbonate synthesized from the above monomer units.
31.14
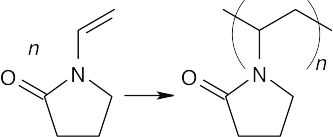
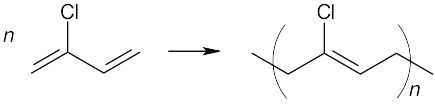 (b)
(b)
Both of these polymers are chain-growth polymers. To draw the polymer in (a), break the double bond, and draw its extensions, one on each side of the former double bond. In (b), break both double bonds and draw extensions at both ends of the former diene. The remaining double bond migrates to a position between the double bonds of the former diene.
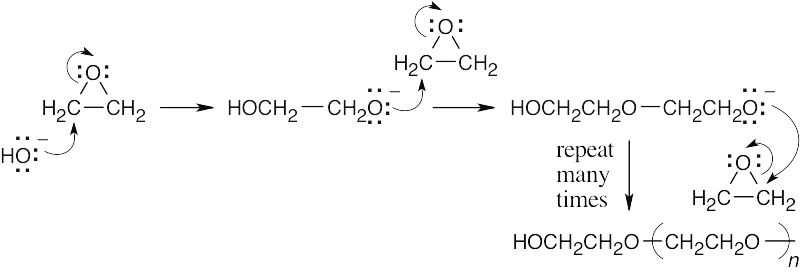 Mechanism Problems 31.15
Mechanism Problems 31.15
31.16 (a)The diamine is formed by an electrophilic aromatic substitution reaction of formaldehyde with two equivalents of aniline.
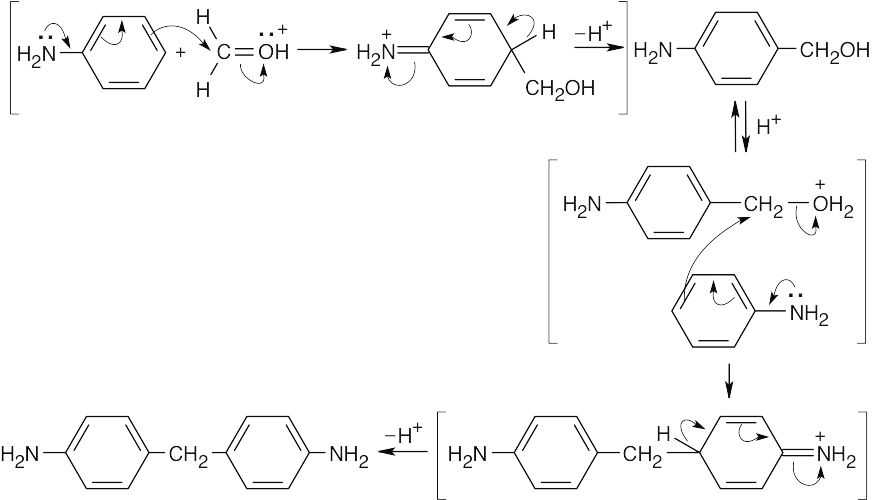
- The diamine reacts with two equivalents of phosgene.
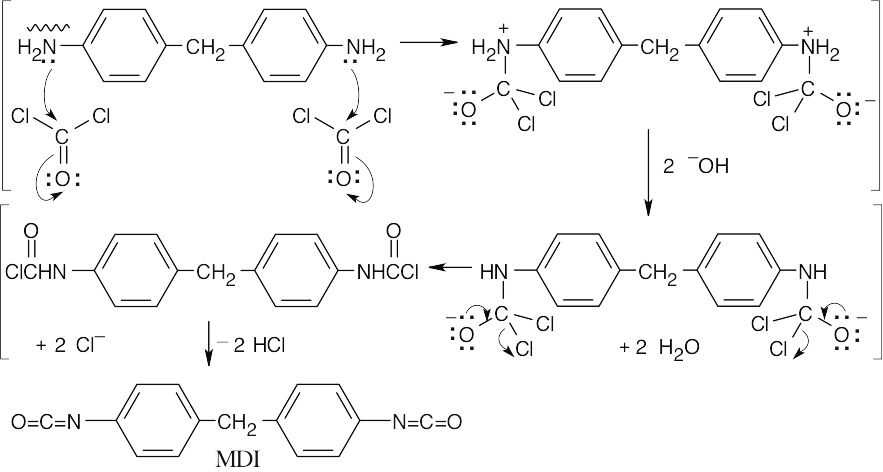
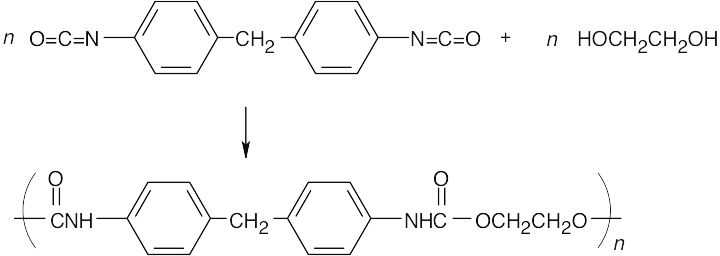
31.17
31.18 Step 1: Polystyrene and the phthalimide combine in an electrophilic aromatic substitution reaction
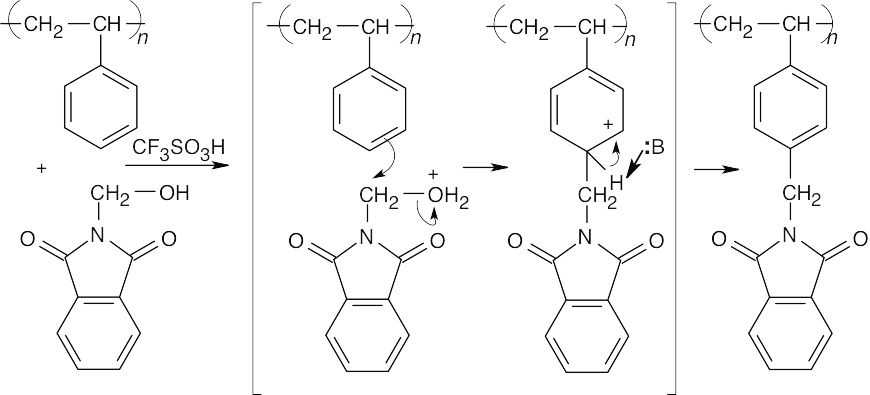
Step 2: The phthalimide is cleaved in a series of steps that involve nucleophilic acyl substitution reactions.
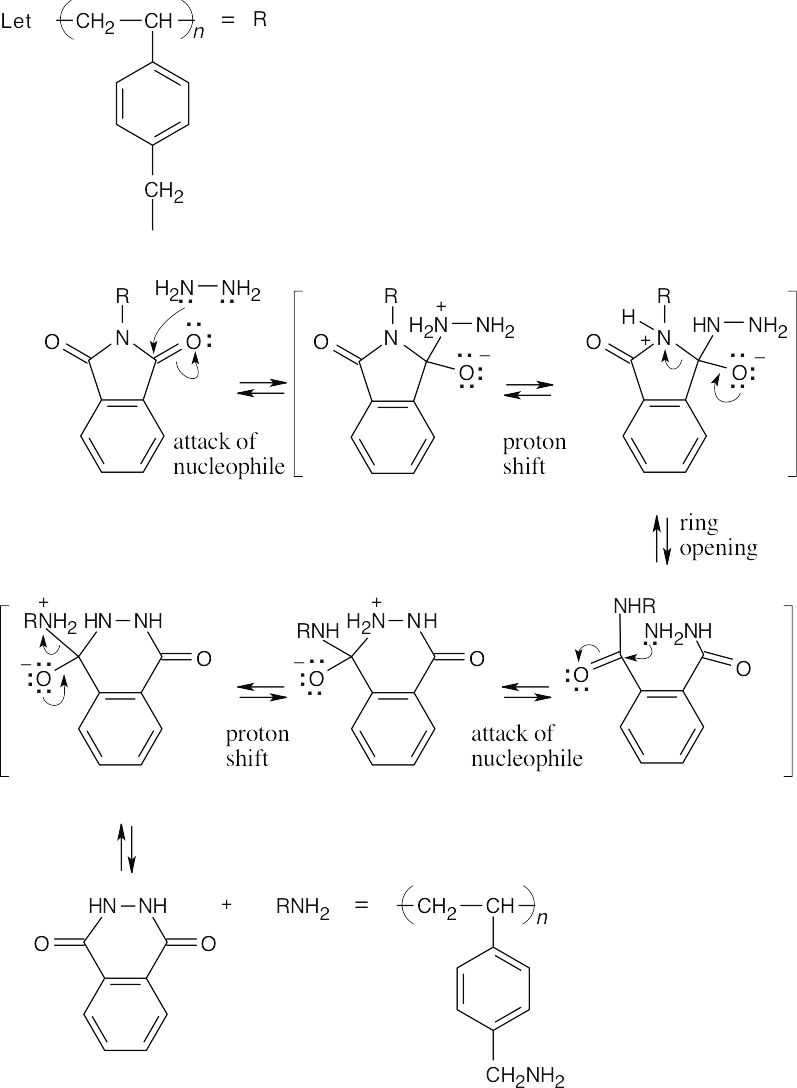
31.19 (a)
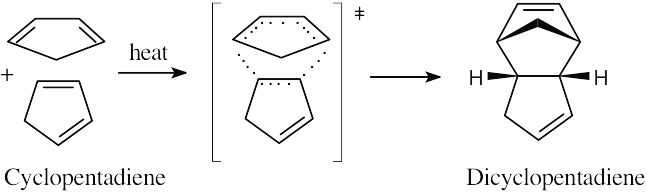
Dicyclopentadiene is formed by a Diels–Alder cycloaddition of two molecules of cyclopentadiene.
(b)
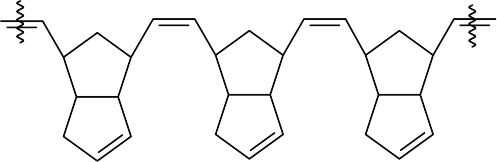
- (c)
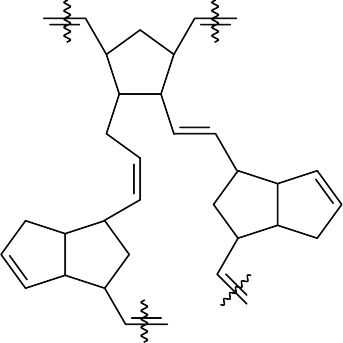
Crosslinking takes place when the remaining double bond of dicyclopentadiene is involved in the polymerization process.
General Problems
31.20
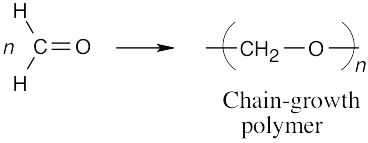 (a)
(a)
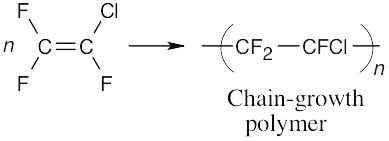 (b)
(b)
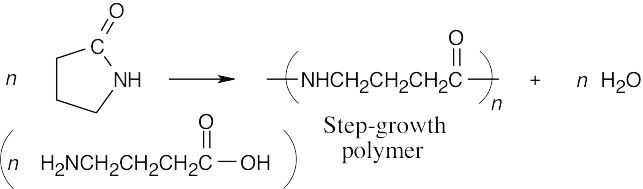 (c)
(c)
(d)
- (e)
31.21 Remember that isotactic polymers have identical groups on the same side of the polymer backbone. Syndiotactic polymers have alternating identical groups along the polymer backbone. Atactic polymers have a random orientation of groups.
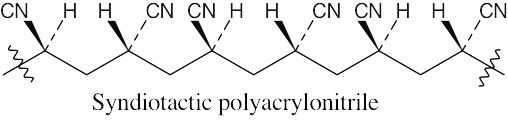 (a)
(a)
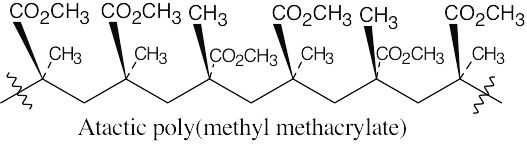 (b)
(b)
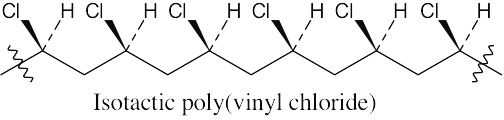 (c)
(c)
31.22
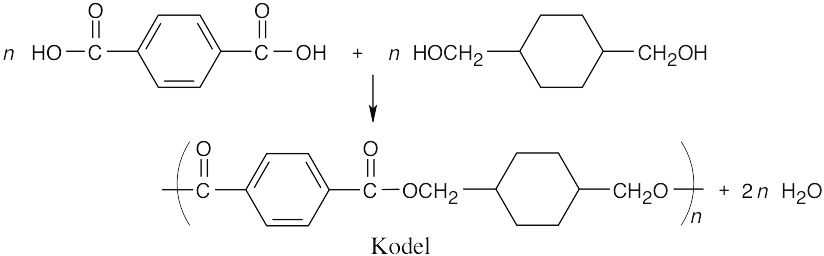
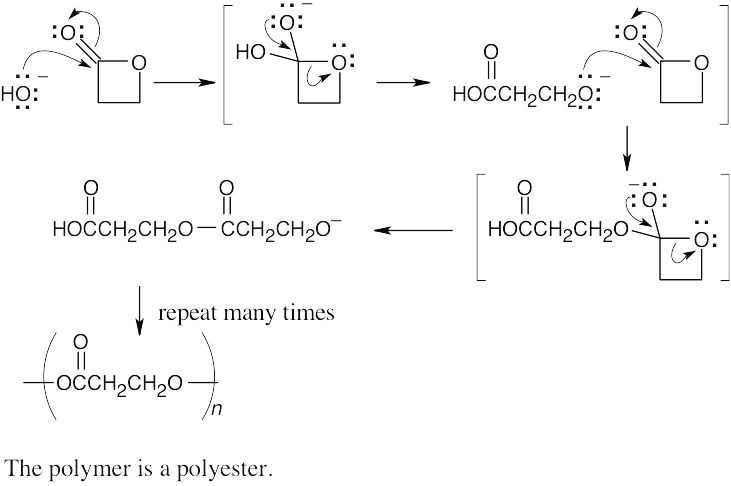
31.23
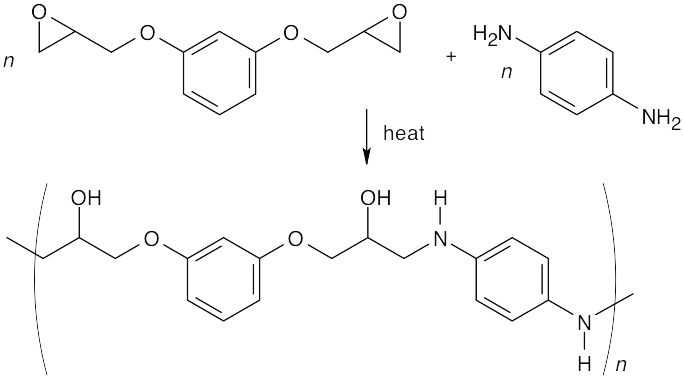
Ring opening of the epoxide occurs by an SN2 pathway at the less substituted epoxide carbon.
31.24
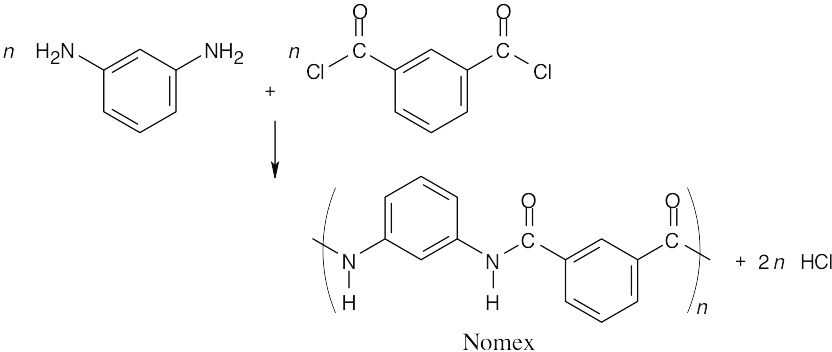
31.25
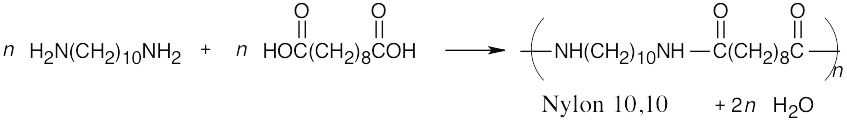
31.26
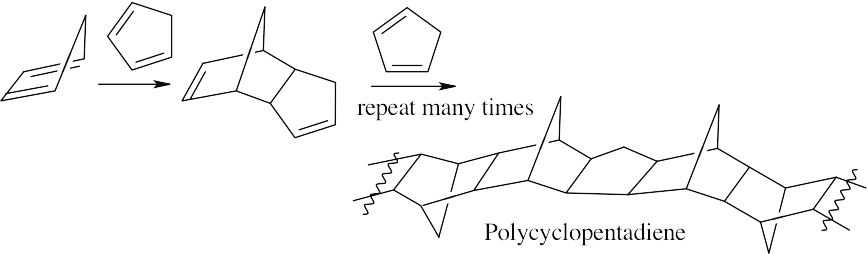
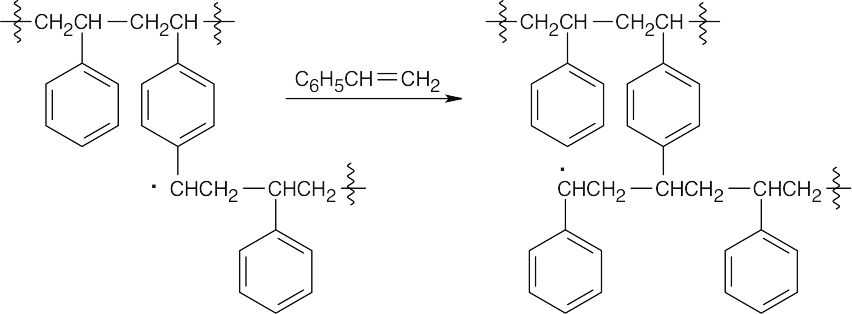
- p-Divinylbenzene is incorporated into the growing polystyrene chain.
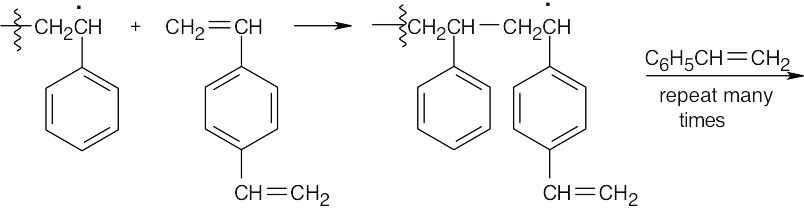
Another growing polymer chain reacts with the second double bond of p-divinylbenzene.
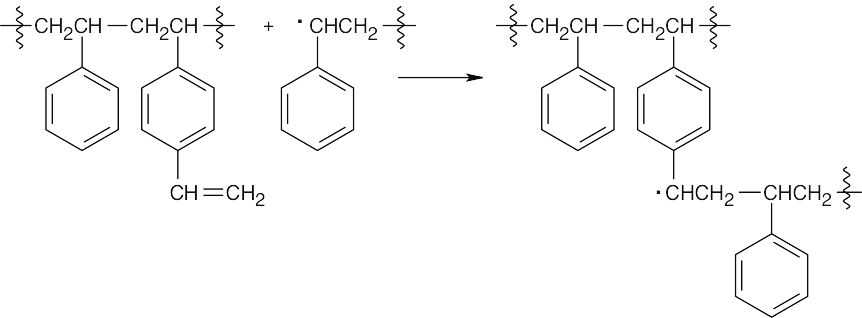
The final product contains polystyrene chains cross-linked by p-divinylbenzene units.
- The white coating on the distillation flask is due to the thermal polymerization of nitroethylene.
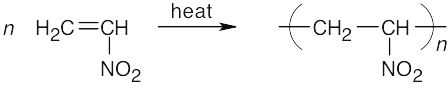
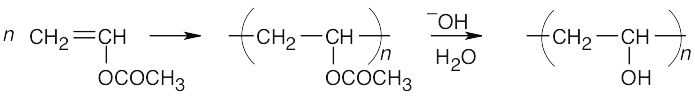
- Poly(vinyl alcohol) is formed by chain-growth polymerization of vinyl acetate, followed by hydrolysis of the acetate groups.
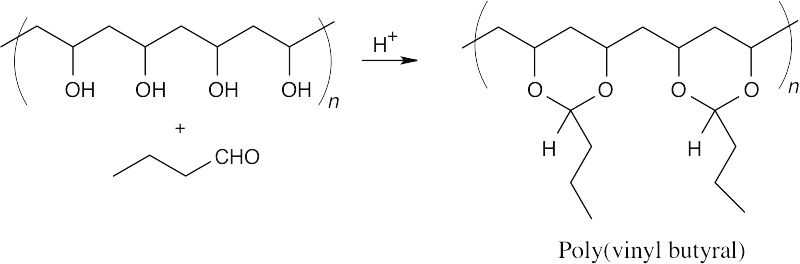
Reaction of poly(vinyl alcohol) with butanal to form the poly(cyclic acetal) produces poly(vinyl butyral).
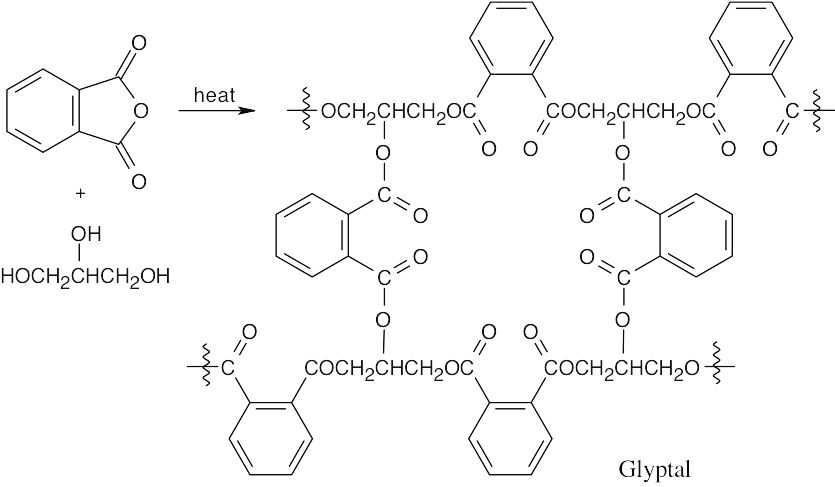
Use of glycerol as a monomer causes the cross-linking that gives Glyptal its strength.
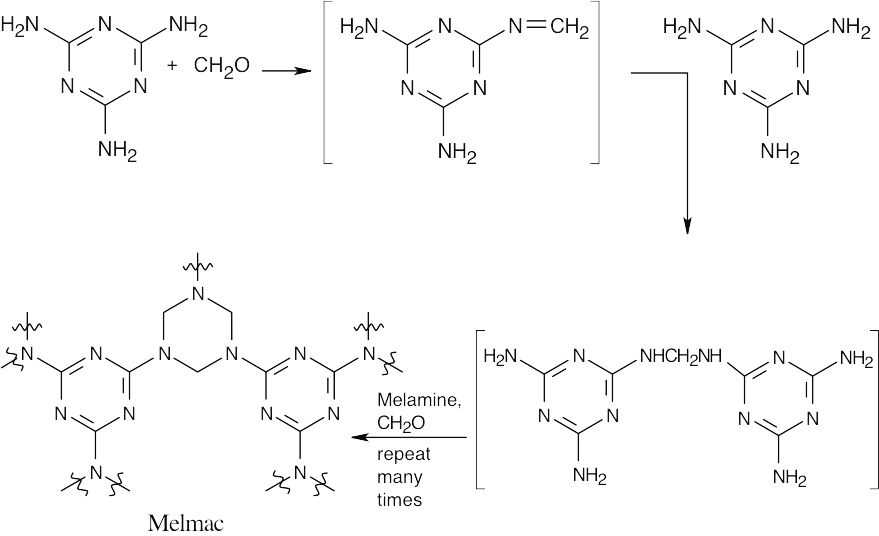
- Repeated nucleophilic acyl substitution reactions result in the formation of Melmac.
31.33 (a)
The prepolymer contains epoxide rings and hydroxyl groups. Copolymerization with a triamine occurs at the epoxide ends of the prepolymer.
(b)
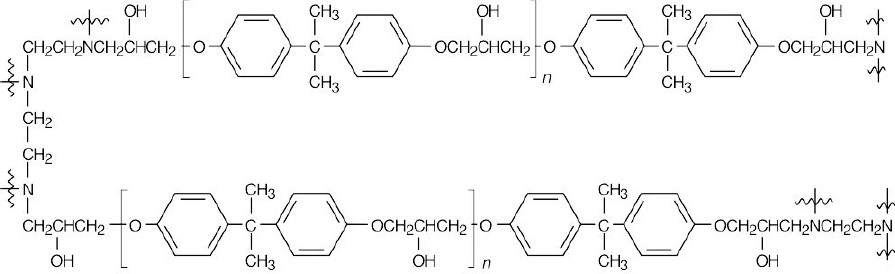
Cross-linking occurs when the triamine opens epoxide rings on two different chains of the prepolymer.
31.34
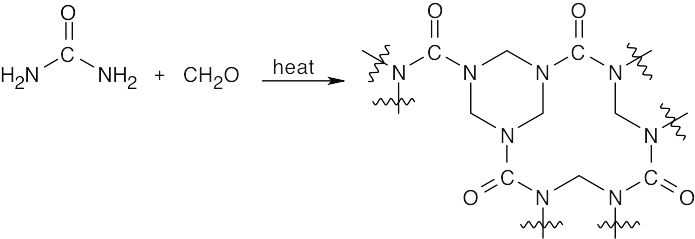
31.35
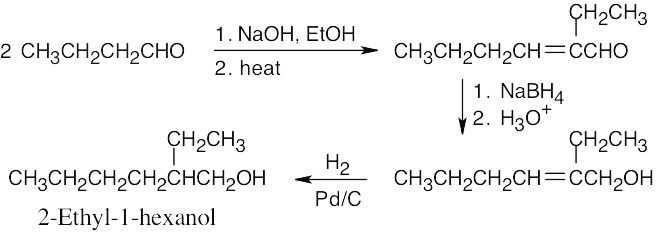
This file is copyright 2023, Rice University. All Rights Reserved.

