55 Current State of the Technology
Brigitte Gallagher; Rachel Falkowski; and Jenna Diemel
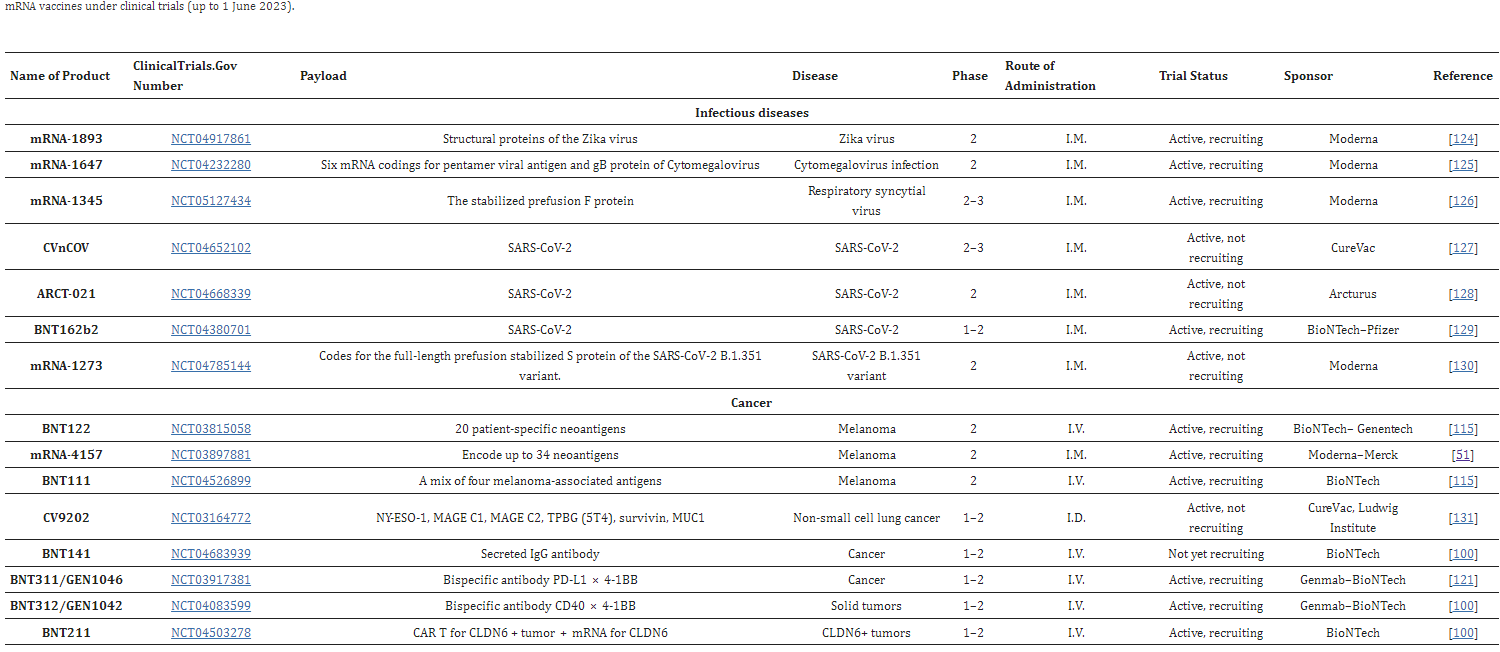
Clinical trials have begun for the development of other vaccines for other diseases like rabies, zika, and several different kinds of cancers (melanomas and lung cancers).
- Table 1 from “Recent advancement in mrna vaccine development and applications,” by N. Al Fayez et al.,
Current commercial availability and any barriers to market entry
- The MRNa technology was highly politicized so many people don’t want to use or disclose MRNa technology in fear of backlash.
- There is also concern for adverse events (anaphylaxis) due to the PEG-lipids which are very common and people can develop sensitivities to that could develop into a more severe reaction.
- Another obstacle is that the vaccines need to be kept very cold (-20C), so developing room temperature stable vaccines are a main goal. Enzymes and temperature changes degrade mRNA, restricting its storage and shelf-life. This demand for ultra-cold storage and specialized transport raises logistical hurdles and expenses for mRNA vaccines.
Since the TED talk, stability has been enhanced for mRNA thermal stability and enzyme resistance. Creating formulations resistant to higher temperatures are vital for future applications and wider distribution. The lipid structures have also been engineered to be stimuli-responsive, breaking down under specific conditions, thus enhancing their versatility. Clinical trials have continued for other vaccines and treatments that have shown some promise.