17 Polymer Information
Introduction of the Polymer Used in HASEL
In the research pertaining to HASEL (Hydraulically Amplified Self-healing Electrostatic) actuators, the polymers typically used are those with good dielectric properties and sufficient flexibility. One common material is a type of silicone rubber, which is often chosen for its elasticity, durability, and excellent insulating characteristics. The specific polymer used in HASEL actuators plays a critical role in their ability to deform and contract in response to electric fields. It should be noted that, depending on the specific research and application, variations and composite materials might be employed to optimize performance and integrate functionalities, such as self-healing or enhanced sensory feedback. For the most accurate information on the materials used in a particular study or version of HASEL actuators, consulting the original research papers or lab publications would be necessary.
Basically, HASELs consist of three parts, which are an elastomeric shell, a flexible electrode based on ionic conductors, and a liquid dielectric layer. The elastomeric shells were made from silicone rubbers such as Ecoflex and polydimethylsiloxane (PDMS, usually Sylgard 184). Stretchable electrodes based on ionic conductors can be synthesized from ionic hydrogels made of gel network and salt aqueous solutions, or ionogels made of gel network and ionic liquids. The liquid dielectric used could be commercially available Envirotemp FR3 (Cargill), which is formulated from vegetable oils and made for use in high-voltage transformers.
Chemical Structures
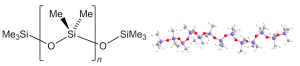
Silicone rubbers are polymers that include silicon together with carbon, hydrogen, and oxygen. The chemical structure of PDMS, for instance, is based on a repeating [-Si(CH₃)₂-O-] unit, with methyl groups attached to the silicon atoms. This structure imparts notable flexibility, thermal stability, and chemical resistance, making it an excellent choice for the elastomeric shells of HASEL actuators.
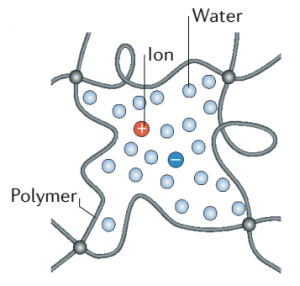
Ionic hydrogels are networks of polymer chains that are highly absorbent in water and can contain salts within their structure. These polymers typically comprise monomers that are cross-linked, with ionic groups attached to the polymer backbone. The salt aqueous solutions incorporated into the hydrogel network facilitate ion conductivity. These hydrogels might contain monomers like acrylamide or acrylic acid, and crosslinkers like N,N’-methylenebisacrylamide, to form a network that can swell in water and allow ionic conduction.
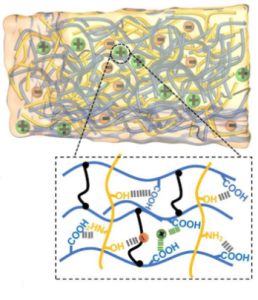
Ionogels combine the properties of ionic liquids, which are salts in a liquid state at room temperature, with a polymer gel network. Generally, the structural framework of ionogels is similar to that of ionic hydrogels but instead of containing water, they are infused with ionic liquids. An ionogel’s polymer network could be based on similar monomers and crosslinkers as hydrogels, while the ionic liquid component typically includes organic cations (such as imidazolium or pyrrolidinium) and various anions (like tetrafluoroborate or hexafluorophosphate). Ionogels display good ionic conductivity and thermal stability due to the non-volatile nature of the ionic liquids.
Physical and Chemical Properties
Chemically, silicone rubbers are polymers made up of siloxane (-Si-O-Si-) chains, with organic groups such as methyl (CH₃) or phenyl (C₆H₅) attached to the silicon atoms. Physically, silicone rubbers are known for their remarkable flexibility and elasticity due to the loose and flexible siloxane backbone. They are able to withstand a wide range of temperatures, from very low to very high, without significant degradation. In terms of their chemical properties, silicone rubbers are inert, non-reactive to most chemicals, and hydrophobic, resisting water and moisture intrusion. They also exhibit excellent electrical insulation properties, which make them useful as dielectric materials in various electrical and electronic applications. In the context of HASEL actuators, their ability to stretch and return to their original shape is crucial for actuation.
Ionic hydrogels are three-dimensional polymer networks that swell in the presence of water or biological fluids while maintaining their structure due to physical or chemical cross-linking. These networks infuse water along with electrolytes (salts), creating a conductive medium that allows for ion transport within the gel. The chemical structure of ionic hydrogels typically includes polar groups which facilitate water absorption and ionic dissociation. Their physical properties are characterized by high water content, soft and rubbery texture, and high permeability for certain molecules and ions. This ability to swell and retain water while conducting ions makes them excellent materials for creating stretchable electrodes in HASEL actuators, as they can simulate the electroactivity and flexibility of biological tissues.
Ionogels are a subcategory of hydrogels that incorporate ionic liquids – salts that are liquid at or around room temperature – into the polymer matrix, instead of water. They combine the mechanical properties of polymer gels with the ionic conductivity and non-volatility of ionic liquids. Ionogels are chemically composed of an intertwined network of organic or inorganic polymers which are imbued with ionic liquids consisting of a large cation, like an imidazolium or ammonium, and an anion, like a halide or an organosulfate. The presence of the ionic liquid gives these gels unique properties such as nearly negligible vapor pressure, remarkable thermal stability, and good ionic conductivity. Their chemical stability and ability to operate over a wide temperature range make ionogels advantageous for electronic applications, like those seen in HASEL actuator electrodes, where maintaining performance in varying environmental conditions is critical.
Benefits
The use of polymeric materials such as silicone rubbers, ionic hydrogels, and ionogels offers numerous benefits across various applications, including HASEL actuators, due to their unique combination of physical and chemical properties.
Silicone rubbers offer significant advantages due to their excellent resilience and flexibility. These polymers can stretch and compress while maintaining their structural integrity, which is critical for replicating the movements of biological muscle in soft robotics. Their thermal stability ensures consistent performance in environments with varying temperatures, from freezing cold to extreme heat. Silicone rubbers are also chemically inert, providing resistance to a wide array of chemicals, oxidation, and UV radiation, which enhances their durability and lifespan. Moreover, their biocompatibility makes them suitable for medical applications such as implants or prosthetics. The hydrophobic nature of silicone rubber also prevents water absorption, making it ideal for applications where moisture resistance is beneficial, especially in electronic devices.
Ionic hydrogels are particularly advantageous in applications that require bio-integration or high water content environments. Their high ionic conductivity, due to the infused electrolytic solutions, enables them to be used effectively as stretchable and flexible electrodes in soft robotic systems like HASEL actuators. The presence of a hydrated network makes ionic hydrogels soft and compliant, which allows them to closely mimic the properties of natural tissues and organs. This is especially useful in biomedical applications where the material is in direct contact with living tissues. Additionally, the porosity of hydrogels permits the diffusion of nutrients and gases, which is beneficial in cell culture and tissue engineering. As sensing elements, they can detect changes in their environment, such as pH or ionic strength, making them useful in diagnostic applications.
Ionogels bring together the mechanical robustness of polymer networks and the electrochemical functionality of ionic liquids. Their ionic conductive properties enable the creation of soft, flexible, and durable electronic components that can sustain a conductive pathway even when deformed. This property is paramount for HASEL actuators, where flexibility and responsiveness to electrical stimuli are required. The almost non-existent vapor pressure of ionogels allows them to operate in a broad range of temperatures without evaporation, which contributes to their longevity and stability in harsh environments. The low flammability and non-volatility of the ionic liquid components make ionogels a safer alternative compared to other conductive materials, especially in wearable electronics and robotics that interface closely with users. The synthesis of ionogels can be modified to include various functional groups, enabling customization for specific needs, such as enhanced adhesion or targeted ion transport, further broadening their application scope.

