5 Scientific Paper on the Topic Written by the Speaker
-
Paper Selection
Microengineered Physiological Biomimicry: Organs-on-Chips
Authors: Dongeun Huh, Yu-suke Torisawa, Geraldine A. Hamilton, Hyun Jung Kima and Donald E. Ingber
-
Summary
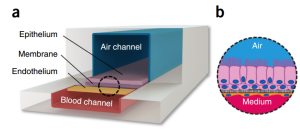
-
Characterization Technique
1. Technique Overview
Microfluidic technique – chemicals, nutrients, hormones, metabolites, cytokines, and physical signals are typically transported across the interfaces of nearby living tissues in our bodies’ organs. These critical structural interactions have been recreated using microengineering technologies, allowing for in vitro investigation of the processes.
The introduction of microfluidic cell-culture devices has changed how we research and manipulate living cells in both 2D and 3D environments. Because of their small size, fluid flow in microfluidic systems is completely laminar (no turbulence), and there is almost no mixing between nearby streams that flow alongside each other within the same hollow channel, as seen in the image below. This novel property has been used to generate abrupt steep gradients on the micrometer scale, delivering chemical gradients across the diameter of a single cell, and to sustain chemical gradients with complex shapes over many hours to days, allowing researchers to study cell motility in response to chemotactic stimuli. It has also been used to investigate more sophisticated cell activities, such as stem cell differentiation, axon guidance, subcellular propagation of biochemical signaling, and embryonic development in 2D culture, as well as to create novel disease models (for example, Parkinson’s disease) (Huh et al., 2012).
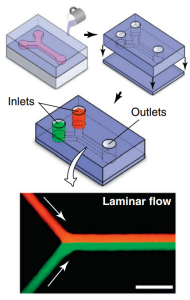
Microfluidic devices are typically created by bonding a PDMS substrate containing microchannel features created by replica molding with a blank PDMS slab. In these microdevices, two fluids (red and green) introduced into independent inlets meet at a Y-junction and enter a straight microchannel in which they flow in adjacent, laminar streams without mixing (arrows indicate flow direction).
Microfluidic networks have been implanted directly into cell-laden hydrogels, allowing for effective convective transport of nutrients and other soluble cues throughout the 3D scaffold. Viable 3D tissue constructs have been created using calcium alginate hydrogels seeded with primary chondrocytes and hepatocytes encapsulated in agarose (Huh et al., 2012).
2. Information Provided by the Technique
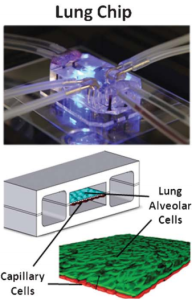
Microfabricating a flexible PDMS device resulted in a living replica of the human alveolar–capillary interface. This was accomplished by cutting a central microfluidic channel horizontally with a porous flexible PDMS membrane, covering it with ECM, and growing human alveolar epithelial cells on one side and pulmonary microvascular endothelial cells on the other. A thin (10 mm) PDMS membrane coated with ECM was extended horizontally across the middle of the main microchannel, containing an ordered array of microengineered pores (10 mm in diameter and 10 mm apart). Human alveolar epithelial cells were cultivated on one side of the porous membrane and covered with air, while human lung capillary endothelial cells were cultured on the other side of the membrane and covered in a flowing media. These tissues were also subjected to physiological mechanical forces that simulate breathing and blood flow by providing cyclic suction to the hollow side chambers and flowing medium through the capillary channel, as depicted in the figure below.
While significant progress has been made in creating the microengineered tissue and organ models detailed in the study, it is still very difficult to integrate several organ chips in a medically plausible manner to represent the entirety of human physiology. It will be necessary to develop “plug-and-play” instrumentation that can support the fluid connections of multiple chips and integration in a way that is physiologically relevant. It should also have systems in place for sample collection and analysis, real-time control, monitoring, visualization, and feedback.
3. Importance to the Technology
These models may affect target validation and lead compound selection and prioritization, among other phases of the drug development process (e.g., by giving enhanced mechanistic insight in a human-relevant system). Furthermore, groups of various organ chips connected by microfluidic connections may offer a means of simulating the physiological interactions between various organs (for example, connecting the liver and the stomach to learn more about the interactions between transporters and drug-metabolizing enzymes), which is essential for identifying the characteristics of drug ADMEs (Huh et al., 2012).
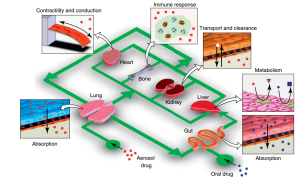
The organ-on-a-chip concept. As shown in this example, an integrated system of microengineered organ mimics (lung, heart, gut, liver, kidney and bone) can be used to study the absorption of inhaled aerosol drugs (red) from the lung to microcirculation, as well as to measure their cardiotoxicity (e.g. changes in heart contractility or conduction), transport and clearance in the kidney, metabolism in the liver, and immune-cell contributions to these responses. Drug substances (blue) also can be introduced into the gut compartment to investigate interplay between orally administered drugs and molecular transporters and metabolizing enzymes expressed in the various organs.
Media Attributions
- Benam, K. H., Villenave, R., Lucchesi, C., Varone, A., Hubeau, C., Lee, H. H., … & Ingber, D. E. (2016). Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nature methods, 13(2), 151-157.
- Huh, D., Hamilton, G. A., & Ingber, D. E. (2011). From 3D cell culture to organs-on-chips. Trends in cell biology, 21(12), 745-754.
- Huh, D., Torisawa, Y. S., Hamilton, G. A., Kim, H. J., & Ingber, D. E. (2012). Microengineered physiological biomimicry: organs-on-chips. Lab on a Chip, 12(12), 2156-2164.
- Huh, D., Hamilton, G. A., & Ingber, D. E. (2011). From 3D cell culture to organs-on-chips. Trends in cell biology, 21(12), 745-754.

