45 Conclusion
ajhodge2; mmdingle; and tmward2
In conclusion, Kaityln Sadtler’s research focuses on harnessing the power of our immune system to allow faster wound healing and tissue regeneration. Just like vaccines cause our bodies to fight disease, Kaitlyn Sadtler is exploring how to teach our immune system to promote tissue growth and repair. By learning and understanding the role of immune cells, specifically helper T cells, in wound healing, we can create materials that guide our body’s natural healing processes without leaving scars or compromising functionality.
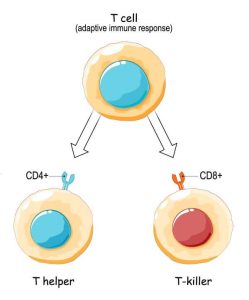
Scaffolds are materials that can interact with our immune system to produce an optimal environment for tissue regeneration. Similar to a theatrical production with its stage, cues, and props, these scaffolds orchestrate a complex interplay of cells and signals to promote specific tissue outcomes. By adjusting these materials, Kaitlyn Sadtler aims to construct scar-proof bandages, moldable muscle fillers, and innovative wound-healing solutions.
The idea of using materials such as scaffolds to instruct our immune system to promote tissue regeneration represents a transformative shift in medical research and public perception. Within the medical field, this research develops new avenues for creating advanced therapies and treatments that influences the body’s natural healing mechanisms. By looking closely at the immune systems interactions and tissue regeneration, scientists are exploring new ways to accelerate wound healing, reduce scarring, and potentially restore damaged tissues and organs. This has lead to collaborative efforts among researchers. clinicians, and industry partners to translate these discoveries into real applications.
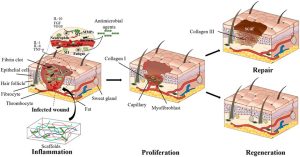
This research has impacted public perception by reshaping how people view the potential medical interventions. This idea leads to a desire for natural and personalized approaches to healthcare. It offers hope for improved outcomes following surgeries, injuries, or chronic conditions, encouraging optimism about the future of medicine and our ability to heal and recover more effectively.
The versatile polymer that we studied, Poly(beta-amino-ester) (PBAE), has surfaced as an important element in the field of biomaterials. PBAE has unique properties that contributes to various biomedical applications. PBAE’s tunable degradation kinetics along with biocompatibility make it attractive for drug delivery systems and tissue engineering scaffolds. The incorporation of PBAE into targeted drug delivery systems highlights its role in enhancing therapeutic efficacy while reducing cytotoxicity. Furthermore, PBAE’s scaffold-forming capabilities facilitate tissue regeneration by providing a conducive environment for cell adhesion, proliferation, and differentiation. Looking in the future, the trajectory of PBAE technology points towards continuing advancements, including refined synthesis techniques, integration with emerging technologies, as well as ethical considerations involving patient privacy and equitable access. Collaborative interdisciplinary efforts will be critical in driving further research and innovation, placing PBAE as a cornerstone in the advancement of personalized and effective biomedical therapies.
Although instant gratification regarding healing may not be met, these advancements can pave the way for practical applications that leverage our body’s defenses to facilitate regeneration. Through ongoing research and innovation, we can wish to control the regenerative abilities observed in nature. Potentially reaching a point where our healing capabilities rival those of amazing organisms like a salamander or lizard.
Media Attributions
- CD4-CD8
- fbioe-10-841583-g001

