6.3 Development of Quantum Theory
Learning Objectives
By the end of this section, you will be able to:
- Extend the concept of wave–particle duality that was observed in electromagnetic radiation to matter as well
- Understand the general idea of the quantum mechanical description of electrons in an atom, and that it uses the notion of three-dimensional wave functions, or orbitals, that define the distribution of probability to find an electron in a particular part of space
- List and describe traits of the four quantum numbers that form the basis for completely specifying the state of an electron in an atom
Bohr’s model explained the experimental data for the hydrogen atom and was widely accepted, but it also raised many questions. Why did electrons orbit at only fixed distances defined by a single quantum number n = 1, 2, 3, and so on, but never in between? Why did the model work so well describing hydrogen and one-electron ions, but could not correctly predict the emission spectrum for helium or any larger atoms? To answer these questions, scientists needed to completely revise the way they thought about matter.
Behavior in the Microscopic World
We know how matter behaves in the macroscopic world—objects that are large enough to be seen by the naked eye follow the rules of classical physics. A billiard ball moving on a table will behave like a particle: It will continue in a straight line unless it collides with another ball or the table cushion, or is acted on by some other force (such as friction). The ball has a well-defined position and velocity (or a well-defined momentum, p = mv, defined by mass m and velocity v) at any given moment. In other words, the ball is moving in a classical trajectory. This is the typical behavior of a classical object.
When waves interact with each other, they show interference patterns that are not displayed by macroscopic particles such as the billiard ball. For example, interacting waves on the surface of water can produce interference patters similar to those shown on Figure 6.13. This is a case of wave behavior on the macroscopic scale, and it is clear that particles and waves are very different phenomena in the macroscopic realm.

As technological improvements allowed scientists to probe the microscopic world in greater detail, it became increasingly clear by the 1920s that very small pieces of matter follow a different set of rules from those we observe for large objects. The unquestionable separation of waves and particles was no longer the case for the microscopic world.
One of the first people to pay attention to the special behavior of the microscopic world was Louis de Broglie. He asked the question: If electromagnetic radiation can have particle-like character, can electrons and other submicroscopic particles exhibit wavelike character? In his 1925 doctoral dissertation, de Broglie extended the wave–particle duality of light to material particles. He predicted that a particle with mass m and velocity v (that is, with linear momentum p) should also exhibit the behavior of a wave with a wavelength value λ, given by this expression in which h is the familiar Planck’s constant:
This is called the de Broglie wavelength. Unlike the other values of λ discussed in this chapter, the de Broglie wavelength is a characteristic of particles and other bodies, not electromagnetic radiation (note that this equation involves velocity [v, m/s], not frequency [ν, Hz]. Although these two symbols appear nearly identical, they mean very different things). Where Bohr had postulated the electron as being a particle orbiting the nucleus in quantized orbits, de Broglie argued that Bohr’s assumption of quantization can be explained if the electron is considered not as a particle, but rather as a circular standing wave such that only an integer number of wavelengths could fit exactly within the orbit (Figure 6.14).
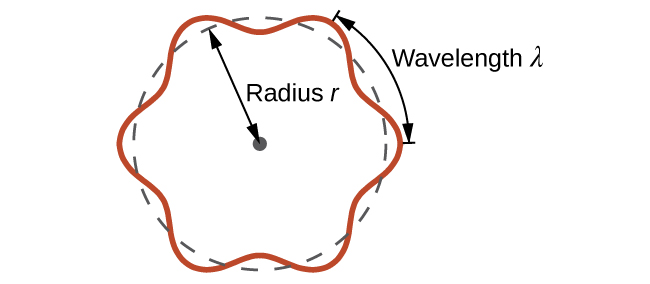
For a circular orbit of radius r, the circumference is 2πr, and so de Broglie’s condition is:
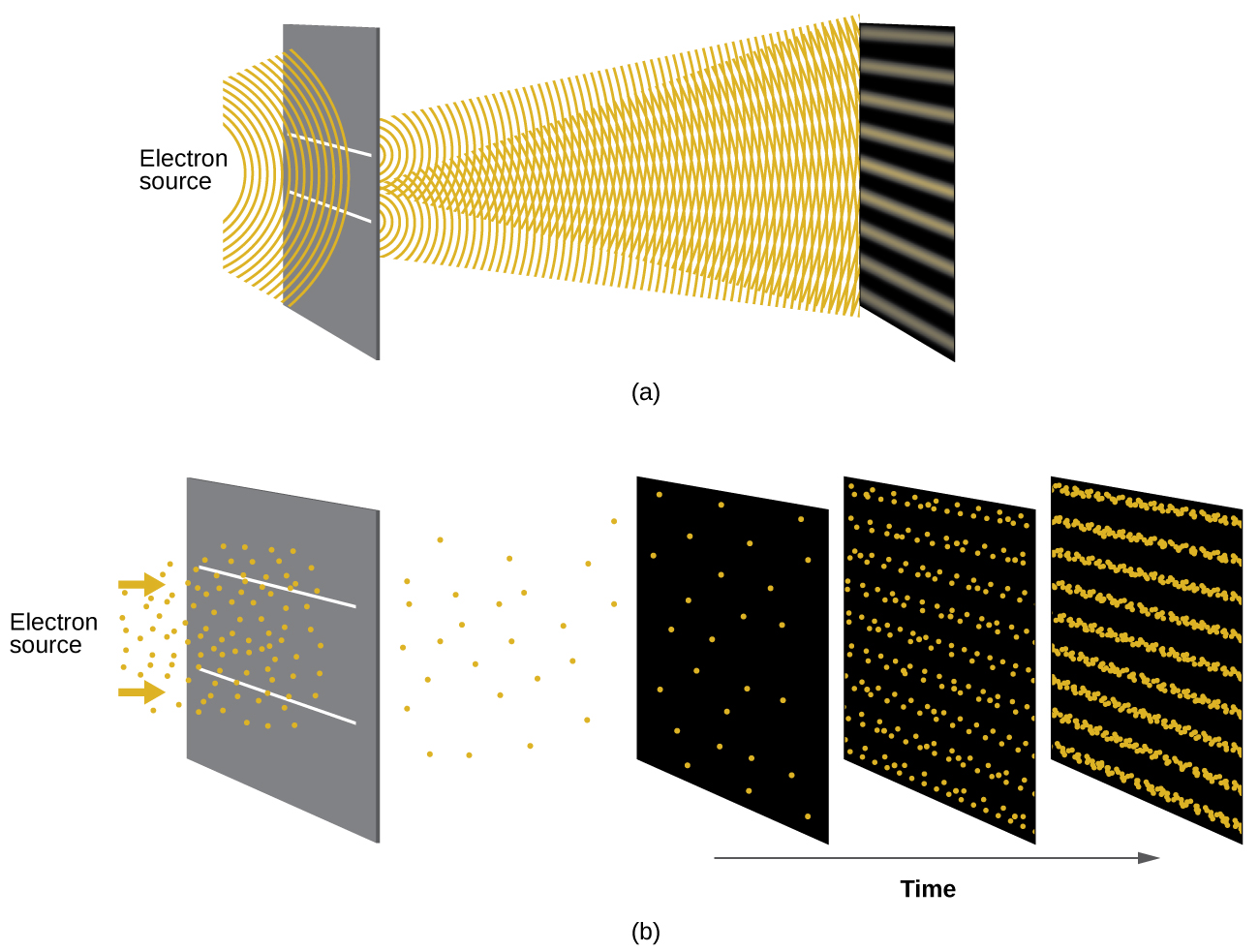
Shortly after de Broglie proposed the wave nature of matter, two scientists at Bell Laboratories, C. J. Davisson and L. H. Germer, demonstrated experimentally that electrons can exhibit wavelike behavior by showing an interference pattern for electrons travelling through a regular atomic pattern in a crystal. The regularly spaced atomic layers served as slits, as used in other interference experiments. Since the spacing between the layers serving as slits needs to be similar in size to the wavelength of the tested wave for an interference pattern to form, Davisson and Germer used a crystalline nickel target for their “slits,” since the spacing of the atoms within the lattice was approximately the same as the de Broglie wavelengths of the electrons that they used. Figure 6.15 shows an interference pattern. It is strikingly similar to the interference patterns for light shown in Electromagnetic Energy for light passing through two closely spaced, narrow slits. The wave–particle duality of matter can be seen in Figure 6.15 by observing what happens if electron collisions are recorded over a long period of time. Initially, when only a few electrons have been recorded, they show clear particle-like behavior, having arrived in small localized packets that appear to be random. As more and more electrons arrived and were recorded, a clear interference pattern that is the hallmark of wavelike behavior emerged. Thus, it appears that while electrons are small localized particles, their motion does not follow the equations of motion implied by classical mechanics, but instead it is governed by some type of a wave equation. Thus the wave–particle duality first observed with photons is actually a fundamental behavior intrinsic to all quantum particles.
Link to Learning
The Quantum–Mechanical Model of an Atom
Shortly after de Broglie published his ideas that the electron in a hydrogen atom could be better thought of as being a circular standing wave instead of a particle moving in quantized circular orbits, Erwin Schrödinger extended de Broglie’s work by deriving what is today known as the Schrödinger equation. When Schrödinger applied his equation to hydrogen-like atoms, he was able to reproduce Bohr’s expression for the energy and, thus, the Rydberg formula governing hydrogen spectra. Schrödinger described electrons as three-dimensional stationary waves, or wavefunctions, represented by the Greek letter psi, ψ. A few years later, Max Born proposed an interpretation of the wavefunction ψ that is still accepted today: Electrons are still particles, and so the waves represented by ψ are not physical waves but, instead, are complex probability amplitudes. The square of the magnitude of a wavefunction, ψ2, describes the probability of the quantum particle being present near a certain location in space. This means that wavefunctions can be used to determine the distribution of the electron’s density with respect to the nucleus in an atom. In the most general form, the Schrödinger equation can be written as:
![]() is the Hamiltonian operator, a set of mathematical operations representing the total energy of the quantum particle (such as an electron in an atom), ψ is the wavefunction of this particle that can be used to find the special distribution of the probability of finding the particle, and E is the actual value of the total energy of the particle.
is the Hamiltonian operator, a set of mathematical operations representing the total energy of the quantum particle (such as an electron in an atom), ψ is the wavefunction of this particle that can be used to find the special distribution of the probability of finding the particle, and E is the actual value of the total energy of the particle.
Schrödinger’s work, as well as that of many other scientists following in their footsteps, is generally referred to as quantum mechanics.
You may also have heard of Schrödinger because of his famous thought experiment. This story explains the concepts of superposition and entanglement as related to a cat in a box with poison.
Understanding Quantum Theory of Electrons in Atoms
In our current quantum mechanical model of the atom, we can no longer accept that electrons travel in set orbits around the nucleus. However, we must still accept the idea of quantized energy levels, as they arose from experimental observations of actual line spectra.
Now, we interpret the energy levels as corresponding to orbitals in the atom. An atomic orbital is interpreted as a general region in an atom within which an electron is most probable to reside. But in truth, they are the wavefunctions that are solutions to the Schrodinger equation. These orbital wavefunctions describe the probability of finding an electron (ψ2, as described above).
As before, the energy levels are labeled with an n value, where n = 1, 2, 3, …. This number, n, is referred to as the principal quantum number. Generally speaking, the energy of an electron in an atom is greater for greater values of n. The principal quantum number is also related to the average distance the electron is from the nucleus. The positively charged protons in the nucleus stabilize the electronic orbitals by electrostatic attraction between the positive charges of the protons and the negative charges of the electrons. So the further away the electron is from the nucleus, the greater the energy it has.
This quantum mechanical model for where electrons reside in an atom can be used to look at electronic transitions, the events when an electron moves from one energy level to another. If the transition is to a higher energy level, energy is absorbed, and the energy change has a positive value. To obtain the amount of energy necessary for the transition to a higher energy level, a photon is absorbed by the atom. A transition to a lower energy level involves a release of energy, and the energy change is negative. This process is accompanied by emission of a photon by the atom.
The principal quantum number, n, is only one of three quantum numbers used to characterize an orbital. Another quantum number is l, the secondary (angular momentum) quantum number. It is an integer that may take the values, l = 0, 1, 2, …, n – 1. This means that an orbital with n = 1 can have only one value of l, l = 0, whereas n = 2 permits l = 0 and l = 1, and so on. Whereas the principal quantum number, n, defines the general size and energy of the orbital, the secondary quantum number l specifies the shape of the orbital. Orbitals with the same value of l define a sublevel.
Orbitals with l = 0 are called s orbitals and they make up the s sublevel. The value l = 1 corresponds to the p orbitals. For a given n, p orbitals constitute a p sublevel (e.g., 3p if n = 3). The orbitals with l = 2 are called the d orbitals, followed by the f-, g-, and h-orbitals for l = 3, 4, and 5.
There are certain distances from the nucleus at which the probability density of finding an electron located at a particular orbital is zero. In other words, the value of the wavefunction ψ is zero at this distance for this orbital. Such a value of radius r is called a node.
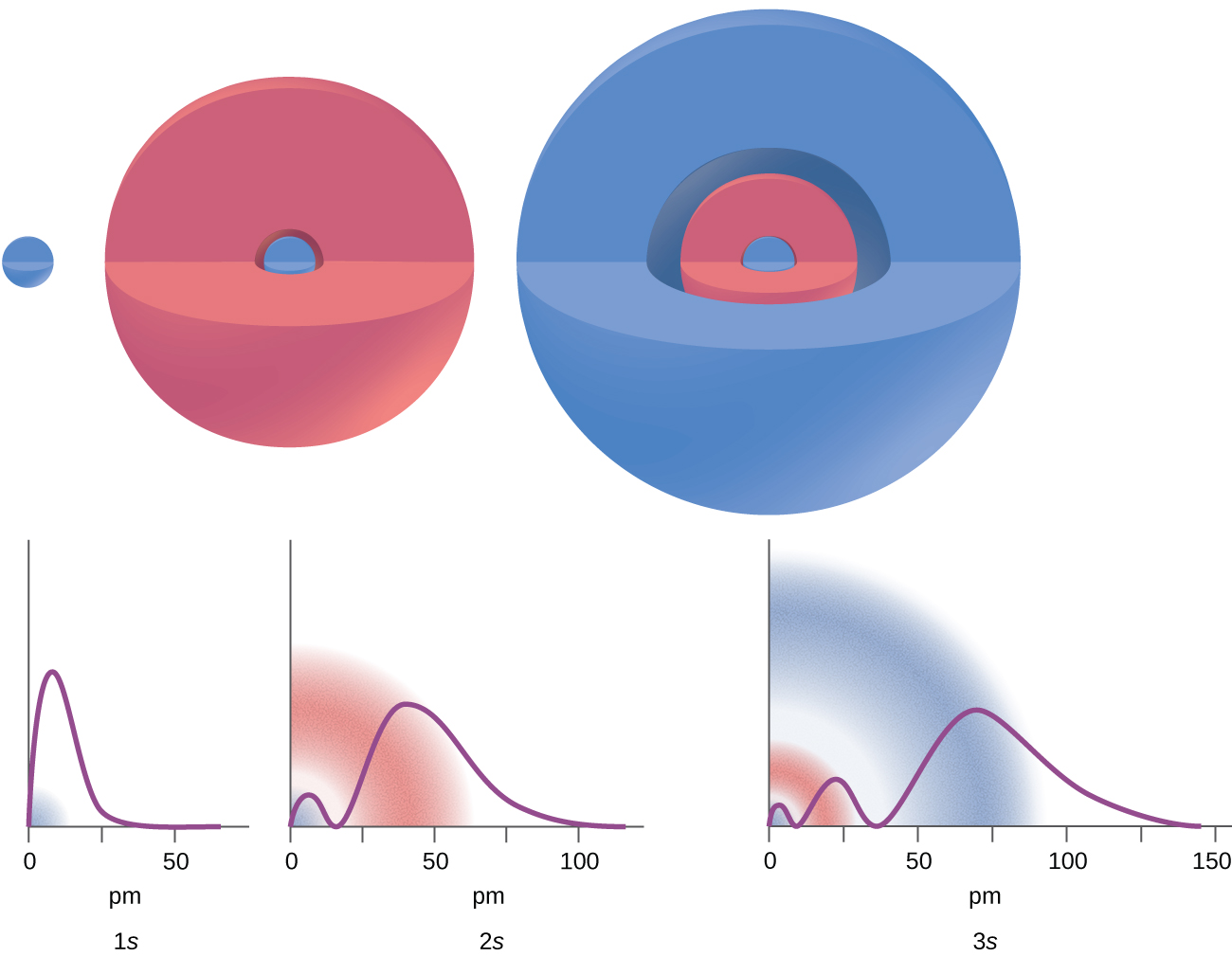
Consider the examples in Figure 6.16. The orbitals depicted are of the s type, thus l = 0 for all of them. It can be seen from the graphs of the probability densities that there zero nodes for 1s (n = 1), 1 node for 2s, and 2 nodes for the 3s orbitals.
The electron density distribution of an s orbital is spherical and a p orbital has a dumbbell shape. The d and f orbitals are more complex. These shapes represent the three-dimensional regions within which the electron is likely to be found.
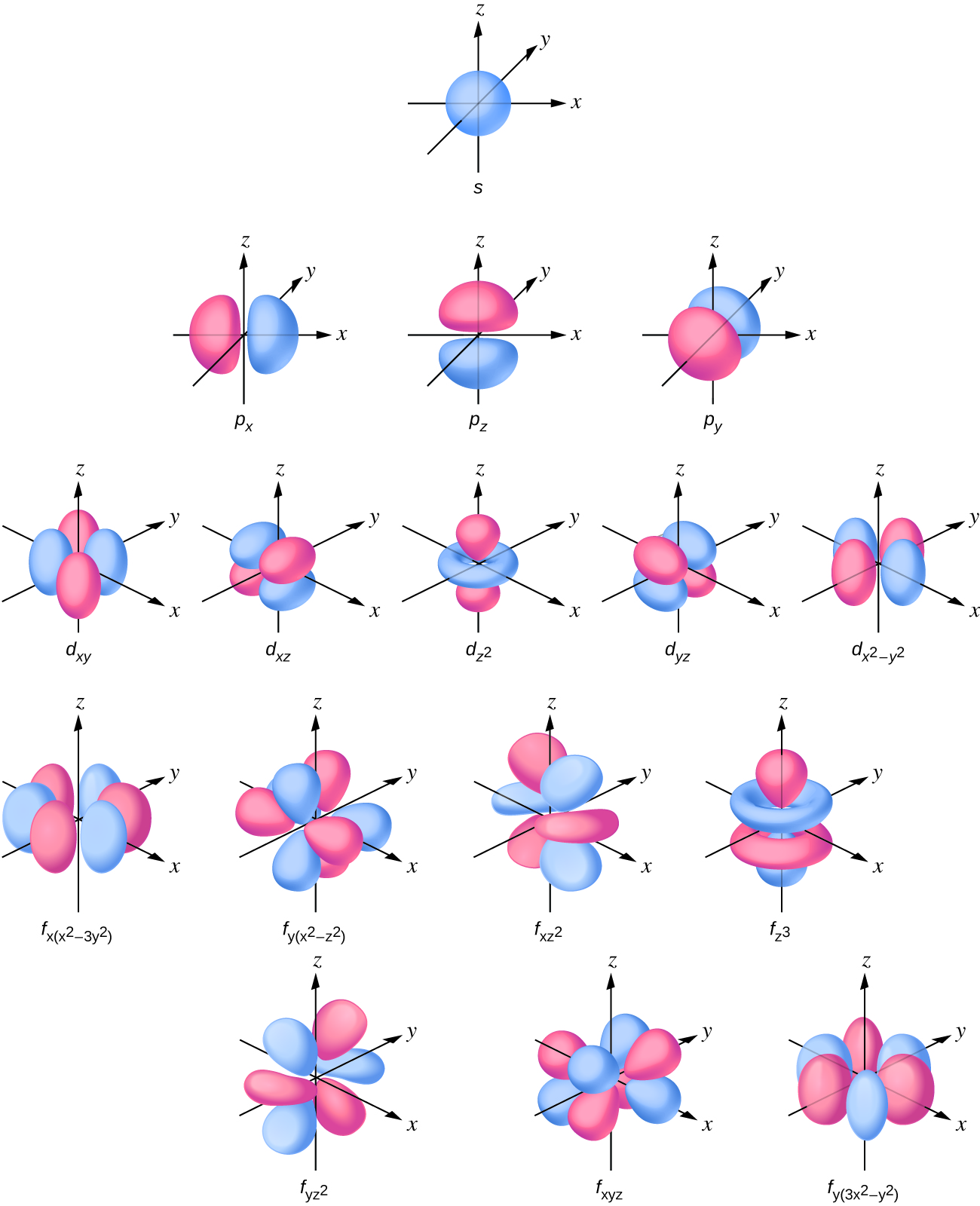
The magnetic quantum number, ml, specifies the relative spatial orientation of a particular orbital. Generally speaking, ml can be equal to –l, –(l – 1), …, 0, …, (l – 1), l. The total number of possible orbitals with the same value of l (that is, in the same sublevel) is 2l + 1. Thus, there is one s-orbital in an s sublevel (l = 0), there are three p-orbitals in a p sublevel (l = 1), five d-orbitals in a d sublevel (l = 2), seven f-orbitals in an f sublevel (l = 3), and so forth. The principal quantum number defines the general value of the electronic energy. The angular momentum quantum number determines the shape of the orbital. And the magnetic quantum number specifies orientation of the orbital in space, as can be seen in Figure 6.17.
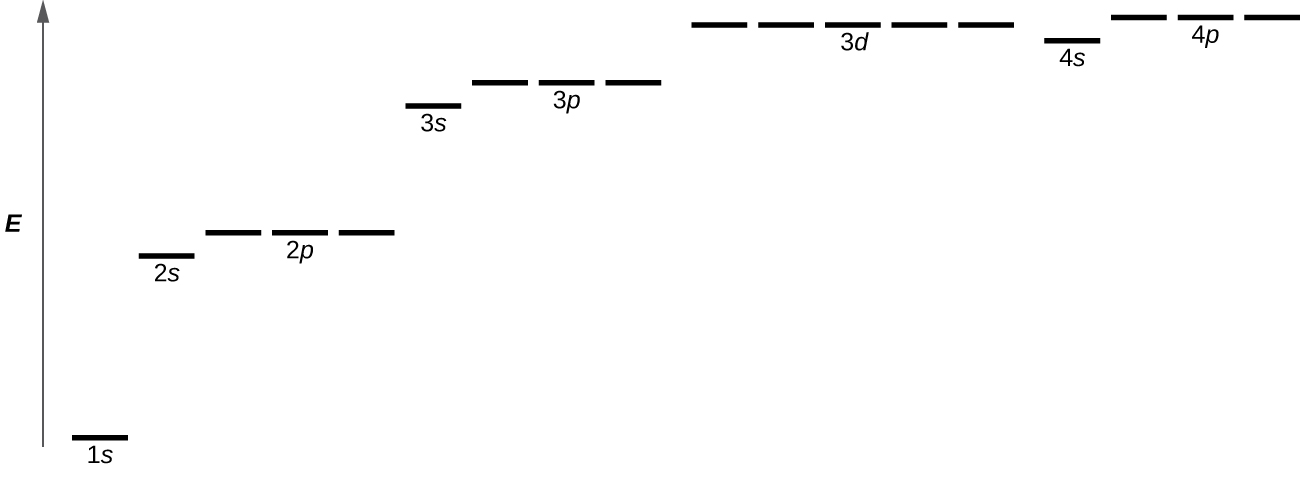
Figure 6.18 illustrates the energy levels for various orbitals. The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. The letter in the orbital name defines the sublevel with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for d orbitals. Finally, there are more than one possible orbitals for l ≥ 1, each corresponding to a specific value of ml. In the case of a hydrogen atom or a one-electron ion (such as He+, Li2+, and so on), energies of all the orbitals with the same n are the same. This is called a degeneracy, and the energy levels for the same principal quantum number, n, are called degenerate orbitals. However, in atoms with more than one electron, this degeneracy is eliminated by the electron–electron interactions, and orbitals that belong to different sublevels have different energies, as shown on Figure 6.18. Orbitals within the same sublevel are still degenerate and have the same energy.
While the three quantum numbers discussed in the previous paragraphs work well for describing electron orbitals, some experiments showed that they were not sufficient to explain all observed results. It was demonstrated in the 1920s that when hydrogen-line spectra are examined at extremely high resolution, some lines are actually not single peaks but, rather, pairs of closely spaced lines. This is the so-called fine structure of the spectrum, and it implies that there are additional small differences in energies of electrons even when they are located in the same orbital. These observations led Samuel Goudsmit and George Uhlenbeck to propose that electrons have a fourth quantum number. They called this the spin quantum number, or ms.
The other three quantum numbers, n, l, and ml, are properties of specific atomic orbitals that also define in what part of the space an electron is most likely to be located. Orbitals are a result of solving the Schrödinger equation for electrons in atoms. The electron spin is a different kind of property. It is a completely quantum phenomenon with no analogues in the classical realm. In addition, it cannot be derived from solving the Schrödinger equation and is not related to the normal spatial coordinates (such as the Cartesian x, y, and z). Electron spin describes an intrinsic electron “rotation” or “spinning.” Each electron acts as a tiny magnet or a tiny rotating object with an angular momentum, or as a loop with an electric current, even though this rotation or current cannot be observed in terms of spatial coordinates.
The magnitude of the overall electron spin can only have one value, and an electron can only “spin” in one of two quantized states. One is termed the α state, with the z component of the spin being in the positive direction of the z axis. This corresponds to the spin quantum number ms = ½. The other is called the β state, with the z component of the spin being negative and ms = -½. Any electron, regardless of the atomic orbital it is located in, can only have one of those two values of the spin quantum number. The energies of electrons having ms = -½ and ms = ½ are different if an external magnetic field is applied.
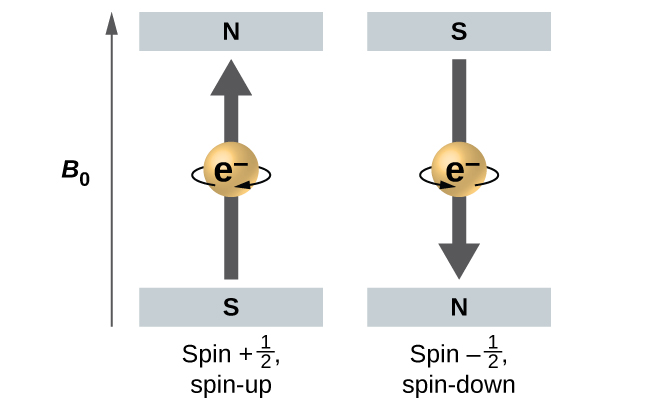
Figure 6.19 illustrates this phenomenon. An electron acts like a tiny magnet. Its moment is directed up (in the positive direction of the z axis) for the ½ spin quantum number and down (in the negative z direction) for the spin quantum number of -½. A magnet has a lower energy if its magnetic moment is aligned with the external magnetic field (the left electron on Figure 6.19) and a higher energy for the magnetic moment being opposite to the applied field. This is why an electron with ms = ½ has a slightly lower energy in an external field in the positive z direction, and an electron with ms = -½ has a slightly higher energy in the same field. This is true even for an electron occupying the same orbital in an atom. A spectral line corresponding to a transition for electrons from the same orbital but with different spin quantum numbers has two possible values of energy; thus, the line in the spectrum will show a fine structure splitting.
The Pauli Exclusion Principle
An electron in an atom is completely described by four quantum numbers: n, l, ml, and ms. The first three quantum numbers define the orbital and the fourth quantum number describes the intrinsic electron property called spin. An Austrian physicist Wolfgang Pauli formulated a general principle that gives the last piece of information that we need to understand the general behavior of electrons in atoms. The Pauli exclusion principle can be formulated as follows: No two electrons in the same atom can have exactly the same set of all the four quantum numbers. What this means is that two electrons can share the same orbital (the same set of the quantum numbers n, l, and ml) only if their spin quantum numbers ms have different values. Since the spin quantum number can only have two values, ±½, no more than two electrons can occupy the same orbital (and if two electrons are located in the same orbital, they must have opposite spins). Therefore, any atomic orbital can be populated by only zero, one, or two electrons.
The properties and meaning of the quantum numbers of electrons in atoms are briefly summarized in Table 6.1.
| Quantum Numbers, Their Properties, and Significance | |||
|---|---|---|---|
| Name | Symbol | Allowed values | Physical meaning |
| principal quantum number | n | 1, 2, 3, 4, …. | energy level, the general region for the value of energy for an electron on the orbital |
| angular momentum or azimuthal quantum number | l | 0 ≤ l ≤ n – 1 | sublevel, the shape of the orbital |
| magnetic quantum number | ml | – l ≤ ml ≤ l | orientation of the orbital |
| spin quantum number | ms | ±½ | direction of the intrinsic quantum “spinning” of the electron |
Table 6.1
Example 6.5
Working with Energy Levels and Sublevels:
Indicate the number of sublevels, the number of orbitals in each sublevel, and the values of l and ml for the orbitals in the n = 4 energy level of an atom.
Solution:
For n = 4, l can have values of 0, 1, 2, and 3. Thus, s, p, d, and f sublevels are found in the n = 4 energy level of an atom. For l = 0 (the s sublevel), ml can only be 0. Thus, there is only one 4s orbital. For l = 1 (p-type orbitals), ml can have values of –1, 0, +1, so we find three 4p orbitals. For l = 2 (d-type orbitals), ml can have values of –2, –1, 0, +1, +2, so we have five 4d orbitals. When l = 3 (f-type orbitals), ml can have values of –3, –2, –1, 0, +1, +2, +3, and we can have seven 4f orbitals. Thus, we find a total of 16 orbitals in the n = 4 energy level of an atom.
Check Your Learning:
Identify the sublevel in which electrons with the following quantum numbers are found: (a) n = 3, l = 1; (b) n = 5, l = 3; (c) n = 2, l = 0.
(a) 3p (b) 5f (c) 2s
Example 6.6
Maximum Number of Electrons:
Calculate the maximum number of electrons that can occupy an energy level with (a) n = 2, (b) n = 5, and (c) n as a variable. Note you are only looking at the orbitals with the specified n value, not those at lower energies.
Solution:
(a) When n = 2, there are four orbitals (a single 2s orbital, and three orbitals labeled 2p). These four orbitals can contain eight electrons.
(b) When n = 5, there are five sublevels of orbitals that we need to sum:
Again, each orbital holds two electrons, so 50 electrons can fit in this energy level.
(c) The number of orbitals in any energy level n will equal n2. There can be up to two electrons in each orbital, so the maximum number of electrons will be 2n2.
Check Your Learning:
If an energy level contains a maximum of 32 electrons, what is the principal quantum number, n?
n = 4
Example 6.7
Working with Quantum Numbers:
Complete the following table for atomic orbitals:
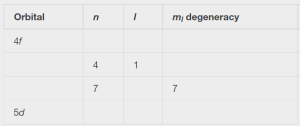
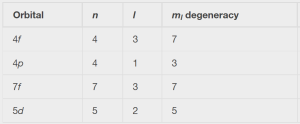
Solution:
The table can be completed using the following rules:
- The orbital designation is nl, where l = 0, 1, 2, 3, 4, 5, … is mapped to the letter sequence s, p, d, f, g, h, …,
- The ml degeneracy is the number of orbitals within an l sublevel, and so is 2l + 1 (there is one s orbital, three p orbitals, five d orbitals, seven f orbitals, and so forth).
Check Your Learning:
How many orbitals have l = 2 and n = 3?
The five degenerate 3d orbitals
Key Concepts and Summary
Macroscopic objects act as particles. Microscopic objects (such as electrons) have properties of both a particle and a wave. Their exact trajectories cannot be determined. The quantum mechanical model of atoms describes the three-dimensional position of the electron in a probabilistic manner according to a mathematical function called a wavefunction, often denoted as ψ. Atomic wavefunctions are also called orbitals. The squared magnitude of the wavefunction describes the distribution of the probability of finding the electron in a particular region in space. Therefore, atomic orbitals describe where electrons in an atom are most likely to be found.
An atomic orbital is characterized by three quantum numbers. The principal quantum number, n, can be any positive integer. The general region for value of energy of the orbital and the average distance of an electron from the nucleus are related to n. Orbitals having the same value of n are said to be in the same energy level. The secondary (angular momentum) quantum number, l, can have any integer value from 0 to n – 1. This quantum number describes the shape or type of the orbital. Orbitals with the same principal quantum number and the same l value belong to the same sublevel. The magnetic quantum number, ml, with 2l + 1 values ranging from –l to +l, describes the orientation of the orbital in space. In addition, each electron has a spin quantum number, ms, that can be equal to ±½. No two electrons in the same atom can have the same set of values for all the four quantum numbers.
Glossary
- secondary (angular momentum) quantum number (l)
- quantum number distinguishing the different shapes of orbitals; it is also a measure of the orbital angular momentum
- atomic orbital
- mathematical function that describes the behavior of an electron in an atom (also called the wavefunction)
- d orbital
- region of space with high electron density that is either four lobed or contains a dumbbell and torus shape; describes orbitals with l = 2.
- degenerate orbitals
- orbitals that have the same energy
- electron density
- a measure of the probability of locating an electron in a particular region of space, it is equal to the squared absolute value of the wave function ψ
- energy level
- atomic orbitals with the same principal quantum number, n
- magnetic quantum number (ml)
- quantum number signifying the orientation of an atomic orbital around the nucleus
- p orbital
- dumbbell-shaped region of space with high electron density, describes orbitals with l = 1
- Pauli exclusion principle
- specifies that no two electrons in an atom can have the same value for all four quantum numbers
- principal quantum number (n)
- quantum number specifying the energy level an electron occupies in an atom
- quantum mechanics
- field of study that includes quantization of energy and wave-particle duality to describe matter
- s orbital
- spherical region of space with high electron density, describes orbitals with l = 0
- spin quantum number (ms)
- number specifying the electron spin direction, either ½ or -½
- sublevel
- atomic orbitals with the same values of n and l
- wavefunction (ψ)
- mathematical description of an atomic orbital that describes the shape of the orbital; it can be used to calculate the probability of finding the electron at any given location in the orbital, as well as dynamical variables such as the energy and the angular momentum

