4.2 Electronic Structure of Alkenes
We saw in the chapter on Structure and Bonding that the carbon–carbon double bond can be described in two ways. In valence bond language (Section 1.8), the carbons are sp2-hybridized and have three equivalent hybrid orbitals that lie in a plane at angles of 120° to one another. The carbons form a σ bond by a head-on overlap of sp2 orbitals and form a π bond by sideways overlap of unhybridized p orbitals oriented perpendicular to the sp2 plane, as shown in Figure 1.15.
In molecular orbital language, interaction between the p orbitals leads to one bonding and one antibonding π molecular orbital. The π bonding MO has no node between nuclei and results from a combination of p orbital lobes with the same algebraic sign. The π antibonding MO has a node between nuclei and results from a combination of lobes with different algebraic signs.
Although essentially free rotation around single bonds is possible (Section 2.7), the same is not true of double bonds. For rotation to occur around a double bond, the π bond must break and re-form (Figure 4.2). Thus, the barrier to double-bond rotation must be at least as great as the strength of the π bond itself, an estimated 350 kJ/mol (84 kcal/mol). Recall that the barrier to bond rotation in ethane is only 12 kJ/mol.
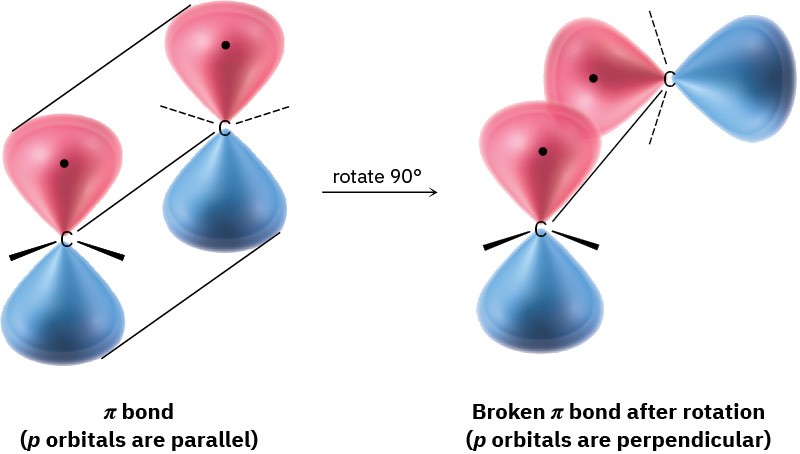 Figure 4.2 The π bond must break for rotation to take place around a carbon– carbon double bond.
Figure 4.2 The π bond must break for rotation to take place around a carbon– carbon double bond.

